Jimma University (JU), Jimma, Ethiopia
The Jimma University Clinical and Nutrition (JUCAN) Research Partnership, based at Jimma University (JU), focuses on malnutrition research in Ethiopia, a significant public health issue. Established in 2008 through collaboration with the University of Copenhagen (KU), JUCAN operates a state-of-the-art body composition (BC) research facility and has conducted numerous impactful nutrition studies. Notably, 11 years ago, the JUCAN team launched the iABC birth cohort at Jimma University Hospital, pioneering the use of air-displacement plethysmography (ADP) technology- a rarity in Sub-Saharan Africa, to study BC changes in children from low-income settings1. The JUCAN center, in collaboration with KU, has provided several postgraduate training opportunities in nutrition, including 11 PhDs that involved seven Ethiopian and four Danish investigators. Among them is Dr. Mike Zangenberg, who completed his PhD on evaluating the appetite test for children with severe acute malnutrition2. During his research, Dr. Mike invited Dr. Øystein H. Johansen to visit JUCAN, where he met Dr. Alemseged Abdissa, the center's director at that time and Prof. Zeleke Mekonnen leading the molecular and parasitology laboratory. At that time Dr. Øystein, who was working at Black Lion and Yekatit 12 Hospitals in Addis Ababa, as part of an exchange programme with Vestfold Hospital Trust in Norway, found Jimma more suitable for conducting the CRYPTO-POC study on cryptosporidiosis in children, funded by The Bill & Melinda Gates Foundation.
Getnet Tesfaw (GT) and Dr. Mike Zangenberg (MZ) discussed the possibility of pursuing a PhD that would build on the CRYPTO-POC3 and Appetite2 studies. GT expressed a keen interest in focusing on gut microbiome analysis for his research. Both MZ and ØHJ also planned to analyze the gut microbiome changes in their respective PhD studies, aiming to use this research to build local capacity. During this process, GT learned that AA, a microbiologist with a focus on malnutrition, had already developed a concept note for a study on the microbiome and malnutrition. Subsequently, AA, MZ, and ØHJ agreed to support GT in conducting his PhD study on gut microbiome analysis. Prof. Henrik Friis one of the key investigators and the founders of JUCAN, introduced the team to Prof. Dennis Sandris Nielson (DSN), who became the main supervisor of GT. GT is now enrolled at the University of Copenhagen as a PhD student.
JU has played a crucial role in the implementation of both CRYPTO-POC3 and the Appetite2 studies by managing the collection of clinical data and stool samples. For CRYPTO-POC3, a microscopic examination of stool samples was carried out at JU molecular and parasitology laboratory. Additionally, JU facilitated the transportation of samples to Vestfold Hospital Trust in Norway, where the total nucleic acids (TNA) extraction of fecal samples was conducted.
University of Copenhagen (KU), Copenhagen, Denmark
After enrolling at KU, Getnet Tesfaw (PhD student) joined a leading team in gut microbiome research at KU, Department of Food Science, Section of Food Microbiology, Gut Health and Fermentation, under the primary supervision of Prof. Dennis Sandris Nielson (DSN). In July 2020, Getnet joined the team of gut microbiome and bioinformatics researchers at KU, where he was hosted for almost 2 years. During this time at KU, GT gained expertise in gut microbiome and bioinformatics analysis, becoming an independent researcher in microbiome and bioinformatics research where the skill and expertise are limited in Ethiopia.
University Bergen (UiB), Bergen, Norway
The University of Bergen (UiB) under the leadership of Prof. Kurt Hanevik, Prof. Nina Langeland, and former PhD student Dr. Øystein H. Johansen has played a key role in the success of this project. In collaboration with Ola Bjørang in the Department of Microbiology, Vestfold Hospital Trust, Tønsberg, total nucleic acids (TNA) were successfully extracted from more than 2000 fecal samples whereafter TNA extracts were sent to the University of Wisconsin (UW), USA for sequencing. Furthermore, Prof. Kurt Hanevik offered mentorship to PhD student (GT) for the success of this project.
University of Wisconsin (UW), USA
Prof. Dawd S. Siraj (DSS), an alumnus of JU, collaborated with Dr. Alemseged Abdissa (AA) on antimicrobial resistance. In 2017, DSS invited AA to the UW for a one-week course at the Quality Improvement Leadership Institute, funded by the African Studies International Collaborator Award. During this visit, DSS introduced AA to Prof. Nasia Safdar (NS), sparking discussions on microbiome research. Led by DSS investigators from JU, KU, UiB, and UW developed a grant proposal that successfully secured funding from the Global Health Institute at the University of Wisconsin. With this grant, UW agreed to host PhD student-GT for three months and cover the cost of microbiome sequencing for the study. Abigail C. Mapes, the project coordinator at UW, has played an invaluable role during project development, implementation, and data sharing. Getnet arrived at UW in February 2020 and began working on the Ethiopian fecal samples after TNA extraction in Norway. However, shortly after his arrival, the COVID-19 pandemic soon erupted, leading to a lockdown that included the UW laboratory where the analysis was supposed to take place. After a month of work and a week under lockdown in Madison, Getnet had to return to Ethiopia. At this point, the project's future seemed uncertain. By March 2020, the pandemic was at its peak in the U.S., with a high death toll and widespread fear. Amid this crisis, Getnet flew back to Ethiopia on a nearly empty plane with just 15 other passengers. Unfortunately, one of the passengers tested positive for COVID-19. After 21 days in quarantine at a hotel in Addis Ababa, Getnet was finally reunited with his family in Ethiopia. The pandemic caused a nearly two-years delay in the project, and sequenced data from the UW finally arrived in October 2021.
Data Sharing
Fortunately, by the time the sequence data arrived at KU, Getnet had already received hands-on training in microbiome and bioinformatics analysis there. The project was particularly challenging to manage and implement due to the need to comply with regulations across three continents and four countries, along with navigating rigorous ethical processes for handling personal and sensitive data. It took a year and a half to secure approval to send stool samples from Ethiopia to Norway, and then TNA extracts from Norway to the U.S.
Sending TNA extracts from Norway, a non-EU country, to the U.S. and receiving the sequence data back in Norway and Denmark involved numerous complex formalities. Questions such as where to store personal and sequence data, who would have access to this data, and who would perform specific tasks required careful planning.
First, JU established a "material transfer agreement" with the three partner universities to facilitate the transfer of stool samples collected from Ethiopia. Next, all universities involved signed an "Overall Collaborative Agreement," which outlined the relationships between the parties, the project's purpose, the flow of materials and data (including stool samples, clinical data, TNA extracts, sequence data, and results), project financing, publication rights, responsibilities, duration, and protocols in case of breach.
Additionally, a "data controller agreement" was signed between KU and UiB to ensure data processing in accordance with General Data Protection Regulation (GDPR) regulations4. UiB and UW also signed a material and data transfer agreement to send TNA extracts and de-identified data from UiB to UW. Finally, JU and KU signed a "guest researcher agreement and data processing agreement" which granted the PhD student access to a secure S-drive on the KU server, provided a dedicated computer for processing personal data, and outlined strict data handling protocols, including using a password-protected USB key, KU email, and KU laptop for all project-related work.
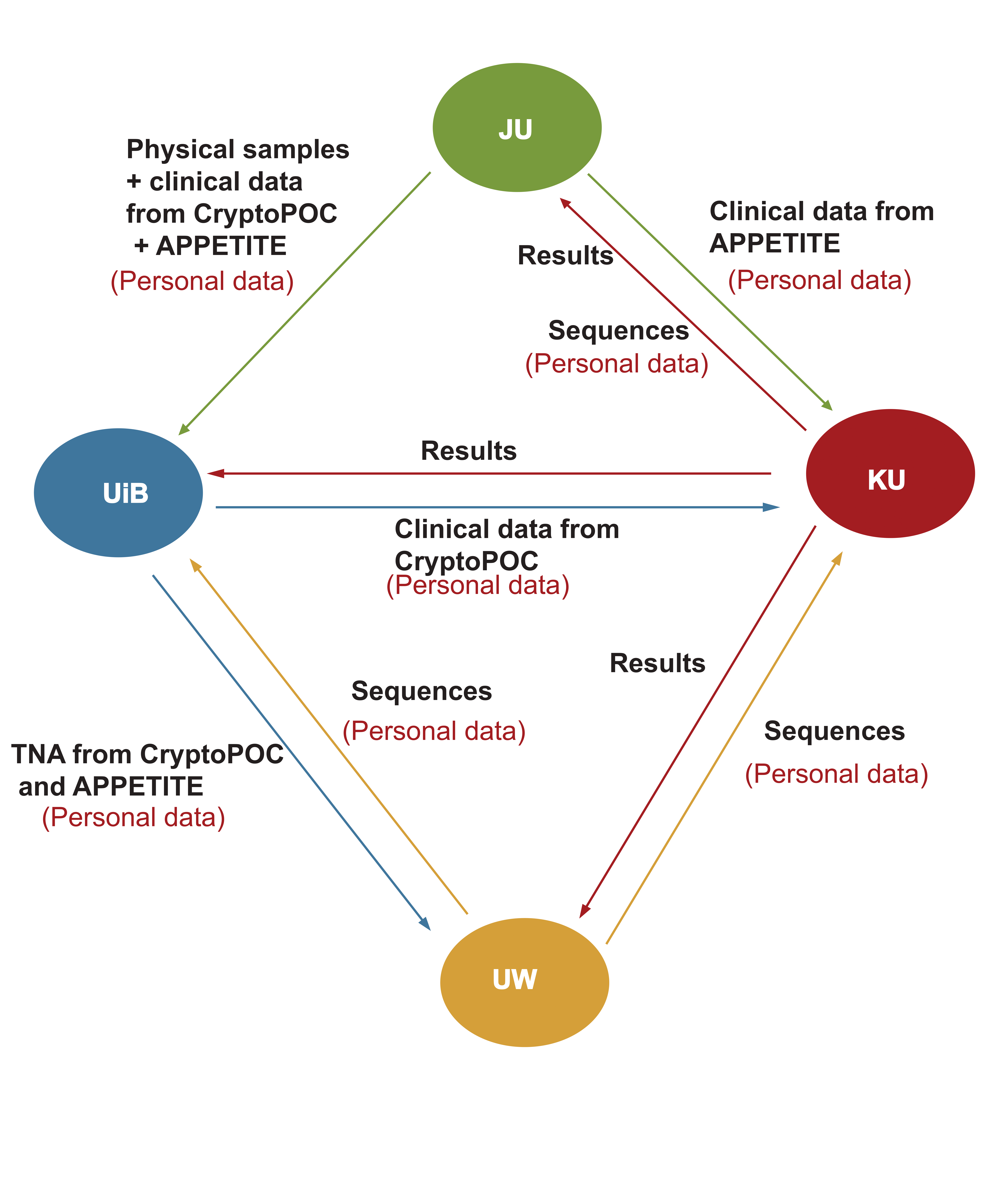
Fig. 1. Data sharing flowchart between participating institutes. TNA= Total Nucleic Acid
In conclusion, although the role of the human microbiome in health and disease is an emerging and crucial area of research, African populations are often underrepresented in microbiome studies due to limited capacity and funding opportunities. This study exemplifies a successful partnership among multiple institutions across three continents. As a spin-off from the malnutrition and diarrhea studies, it presents intriguing findings that could inform program management. The partnership effectively overcame funding limitations by pooling contributions from each institution to conduct microbiome studies in African settings, which remain underserved.
Project Summary
Diarrhea is a major contributor to childhood mortality and morbidity in low- and middle-income countries (LMICs). Childhood mortality attributed to acute diarrhea (AD; < 7 days in duration) has substantially decreased in the last decades due to the well-established oral rehydration therapy. Unfortunately, prolonged (ProD; 7-13 days in duration) and persistent (PD; ≥ 14 days in duration) diarrhea combined (ProPD) remain a significant challenge and are responsible for more than half of the deaths attributed to diarrhea. This is due to the lack of treatment guidelines and insufficient evidence about the etiology of ProPD in children in LMICs.
Analyzing of the gut microbiome (GM) suffering from AD or ProPD and non-diarrheal controls demonstrated that children with AD and ProPD in LMICs exhibit a significant GM imbalance, enriched with putative pathogens such as Escherichia spp., Campylobacter spp. and Streptococcus spp. and lacking crucial gut commensals such as Prevotella copri, Faecalibacterium prausnitzii, and Dialister succinatiphilus compared to non-diarrheal controls. The gut commensals play a key role in the production of short-chain fatty acids (SCFAs) and likely maintaining the GM in a stable state. The GM of children with ProPD is characterized by a more pronounced reduction of gut commensals, indicating a high degree of GM imbalance compared to AD cases. This suggests that the use of targeted dietary supplements to enhance the proliferation of important gut commensals and/or the use of next-generation probiotics might help improve the treatment of both AD and ProPD.
References
- Andersen, G.S., et al. Fat and fat-free mass at birth: air displacement plethysmography measurements on 350 Ethiopian newborns. Pediatric research 70, 501-506 (2011).
- Zangenberg, M., et al. Critical evaluation of the appetite test for children with severe acute malnutrition. Tropical Medicine & International Health 25, 424-432 (2020).
- Johansen, Ø.H., et al. Performance and operational feasibility of two diagnostic tests for cryptosporidiosis in children (CRYPTO-POC): a clinical, prospective, diagnostic accuracy study. The Lancet Infectious Diseases 21, 722-730 (2021).
- Regulation, P. Regulation (EU) 2016/679 of the European Parliament and of the Council. Regulation (eu) 679, 2016 (2016).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in