A Grand Challenge
Published in Cancer, Chemistry, and Pharmacy & Pharmacology
Intracellular macromolecule interactions are of fundamental importance to human physiology and pathology. With the notable exceptions of Venetoclax and molecular glues, the biomedical field has been challenged to develop small molecule based medicines that can modulate macromolecular interactions. Monoclonal antibodies, the other major class of therapeutics, have had great success in targeting serum proteins and cell-surface targets, but are restricted to the extracellular space. Peptides have emerged as a modality that sits between small molecules and antibodies – they possess enough chemical real-estate to engage large interfaces and can be engineered to be cell permeable. Peptide macrocycles can also have enough complexity, rigidity and chameleon-like conformational bias to adapt and serve as programable modalities. As a consequence of the emergence of super-diverse peptide library technologies such as phage-, mRNA-, and DNA-display libraries, as well as affinity selection mass spectrometry (ASMS) methods, the identification of high-affinity peptide ligands against targets of interest is now rarely limiting. Proteolytic instability, another traditional challenge for peptidic therapeutics, is now routinely addressed through macrocyclization and substitution with natural and non-natural residues. Thus, the grand challenge for realizing the therapeutic potential of macrocyclic peptides has become crystal clear – how can we transform high-affinity peptide hits into drugs by simultaneously imparting them with membrane permeability while maintaining drug-like solubilities and solution behavior? Aileron Therapeutics pioneered work in this space using hyper-stabilized a-helical or “stapled peptides” targeting MDM2/MDMX to block the interaction with WT p53 and inspired us to tackle this problem further. In parallel to the work in our paper, FogPharma developed a clinical hyper-stabilized peptide or Helicon™ targeting b-catenin.
Assembling the team:
With the advent of super-diverse peptide library technologies, the “third wave” of peptide therapeutics, was in full swing. During this time, A*STAR (The Agency for Science Technology and Research) made pioneering contributions to the field of stapled peptides, with a team assembled by Sir David Lane (Chief Scientist at A*STAR and Director of the p53 Lab) which included Chris Brown, Fernando Gago, Charlie Johannes, Chandra Verma and the visiting Greg Verdine. Those efforts laid the ground work for the establishment of a Peptide Engineering Program (PEP) led by Charlie Johannes and David Lane. In 2014, Tomi Sawyer joined Merck & Co., Inc from Aileron Therapeutics to spearhead a Peptide Drug Hunter initiative. In that same year, Anthony Partridge joined MSD Singapore and began discussing with Tomi and A*STAR scientists on ways to advance the field of macrocyclic peptides. Those discussions led to a formal collaboration involving Merck & Co., Inc (Tomi Sawyer and team), MSD Singapore (Brian Henry, Anthony Partridge, and team), and several A*STAR institutes, including the p53Lab (David Lane, Chris Brown and team), the Institute of Chemical and Engineering Sciences (Charlie Johannes and team) and the Bioinformatics Institute (Chandra Verma, Raghav Kannan, and team). The collaboration sought to fundamentally understand cellular uptake and how to develop guidelines (including screening tools and design rules) for the development of peptide macrocycles. The team moved quickly by leveraging expertise and infrastructure related to the interaction of MDM2(X) with p53, a protein dubbed “the guardian of the genome” and for which Sir David Lane recently celebrated the 40th-year anniversary of its discovery. The work presented in this paper is a compendium on the application of the knowledge generated.
Our approach and findings:
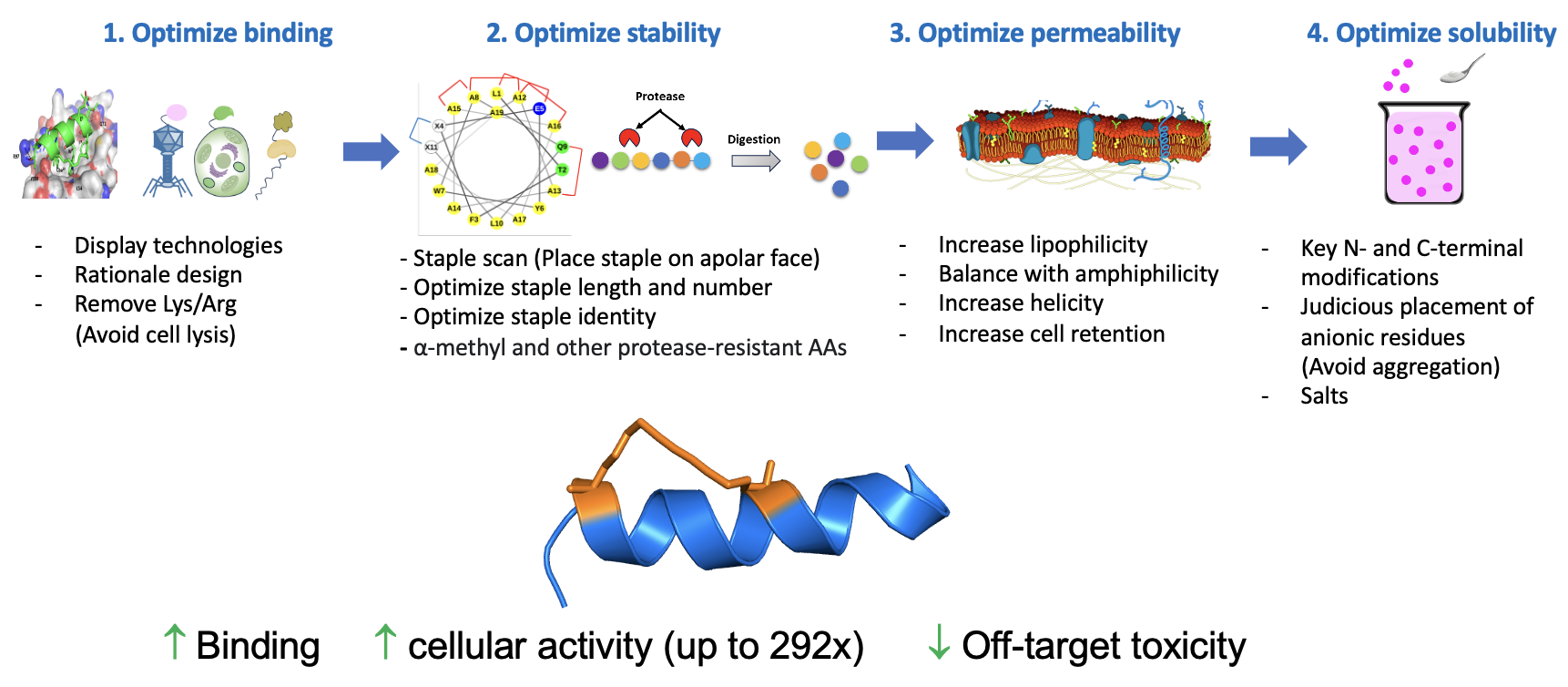
Comparison with and rigorous analysis of pre-existing helical peptides were conducted to evaluate a structurally-diverse series of analogs with respect to target binding, metabolic stability, cell permeability, and solubility using various screening tools. Testing of a library of >350 peptides provided deep insights to the design, structure-property relationships, and ultimately, the identification of significantly improved peptide analogs of Aileron’s clinical candidate ALRN-6924. These design rules have been further applied to an all-D a-helical peptide resulting in improved cell permeability and efficacy. Three key modifications were identified:
- Hydrocarbon staple optimization
- Judicious placement of negative charge
- Poly-alanine incorporation and modifications thereof
These were incorporated in the illustrated workflow.
The study is entitled "Design-Rules for Stapled Peptides with in vivo Activity and their Application to Mdm2/X antagonists" published in Nature Communications, 2024 Jan 12;15(1):489. doi: 10.1038/s41467-023-43346-4.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in