A new copper-catalysed photoinduced radical cyclization of ynamides for the preparation of azetidines
Published in Chemistry

Up to 90% of naturally occurring organic molecules and up to 97% of small organic molecules from the drug industry contain either a carbocycle or a heterocycle. The development of efficient cyclization reactions for the preparation of cyclic molecules is therefore of utmost importance in modern synthetic chemistry. While most cyclization modes have been well developed, the formation of some small rings has however remained elusive and this is the main reason why we became interested in the development of a new radical 4-exo-dig cyclization, which is most certainly the least explored type of cyclization.1,2
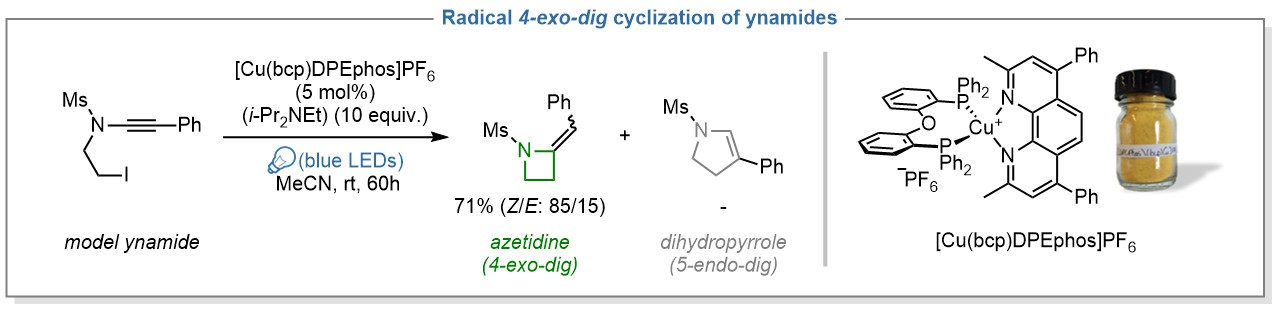
We selected copper-based photoredox catalysis as a sustainable strategy to ensure the highest synthetic utility to the title reaction. For several years, the groups or Pr. Evano and Pr. Moucheron have indeed collaborated to work on the generation of radicals using photoactive copper complexes, which now constitute cheaper and complementary alternatives to more common photocatalysts based on noble metals such as iridium and ruthenium. This interest resulted in the successful activation of carbon – halogen bonds of aryl and alkyl halides using a heteroleptic copper complex, [Cu(bcp)DPEphos]PF6, under irradiation with visible light.3,4 The next step of our research was therefore to bring this chemistry one step further by using this copper-based photocatalyst to promote new and more challenging transformations such as the 4-exo-dig cyclization of ynamides we have recently reported.
The chemistry of ynamides has been extensively studied in our group in the past years and we felt that the strong polarization of these nitrogen-substituted alkynes could be exploited to promote unusual radical cyclizations upon activation under photoredox conditions.5,6 The development of this reaction began with a standard optimization which quickly revealed that the combination of [Cu(bcp)DPEphos]PF6 as photocatalyst and Hünig’s base as sacrificial reductant under visible-light irradiation outperformed all other combinations we have tried, including those based on more common iridium photocatalysts. This system indeed allowed a clean conversion of our model ynamide substrate into the desired azetidine, resulting from a unique 4-exo-dig cyclization, without any traces of the dihydropyrrole that would have resulted from a more common 5-endo-dig pathway.
The next step of our work was to study the scope and limitations of this reaction by subjecting a variety of diversely substituted ynamides to our optimized copper-based photoredox conditions. To make a long story short, we were pleased to realize that most of the ynamides that we had prepared readily cyclized into the desired azetidines in good yields and various substituents were well tolerated at all positions of the starting ynamides. In order to demonstrate the efficiency of the reaction in “real-life” situation, we successfully prepared azetidines derived from much more complex ynamides and also demonstrated that different azetidines could be further post-functionalized thanks to the exocyclic double bond which constitutes a versatile handle towards chemical diversification.
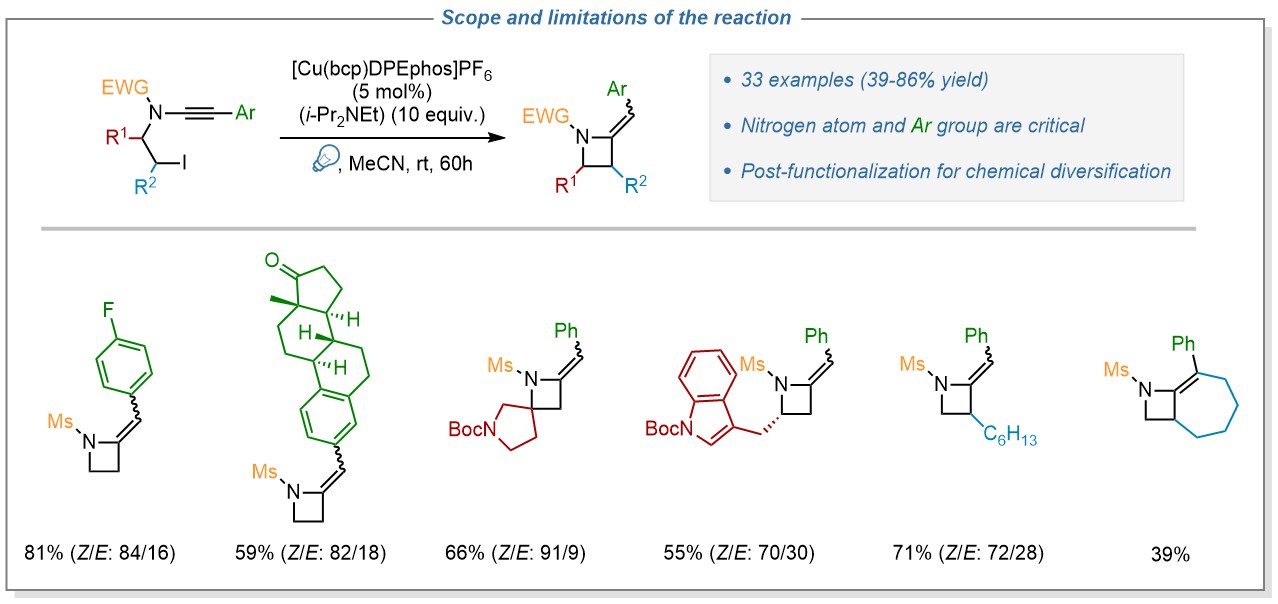
As anticipated when we designed the reaction, the presence of both the nitrogen atom and the aryl substituent on both sides of the triple bond of the starting ynamides is strictly required for the cyclizations to take place. These are indeed critical to ensure a strong enough polarization of the triple bond and to stabilize the transient vinyl radical formed after the 4-exo-dig cyclization step. These effects were further confirmed via a computational study based on DFT calculations which also indicated that this 4-exo-dig cyclization is a kinetically-driven process, as opposed to the complementary thermodynamically favoured 5-endo-dig pathway which was never observed.
As a conclusion, the development of this unusual radical 4-exo-dig cyclization of ynamides was successfully carried out thanks to a close collaboration between the groups of Pr. Evano and Pr. Moucheron at the Université libre de Bruxelles (ULB, Belgium) and the group of Pr. Van Speybroeck at the Universiteit Gent (UGent, Belgium) who was in charge of the computational study. This collaborative environment will be maintained in the near future and we are excited to continue our efforts to further develop new unusual cyclizations based on the photochemistry of copper complexes.
More details on this work can be found here: “A general synthesis of azetidines by copper-catalysed photoinduced anti-Baldwin radical cyclization of ynamides” in Nature communications (DOI: 10.1038/s41467-022-28098-x).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in