A Wearable Device to Protect Dialysis Patients' Lifelines
Published in Bioengineering & Biotechnology and General & Internal Medicine

Chronic kidney disease (CKD) affects millions globally, and for many patients, hemodialysis is the lifeline that sustains them1,2. This process, which cleanses the blood, depends on vascular access (VA)—surgically created connections between veins and arteries or synthetic grafts that provide the high blood flow rates required for dialysis. Yet, these vital access points are vulnerable to failure, often due to narrowing (stenosis) or blockage caused by thrombosis3. Unfortunately, early detection of these failures has been challenging, often leaving patients reliant on reactive, emergency interventions.
Our research introduces a wearable thermal sensor designed to monitor VA patency in real-time, offering a potential paradigm shift in the management of dialysis care. Using principles of thermal anemometry4, this device detects subtle changes in blood flow noninvasively, potentially allowing patients and clinicians to intervene before a complete failure occurs.
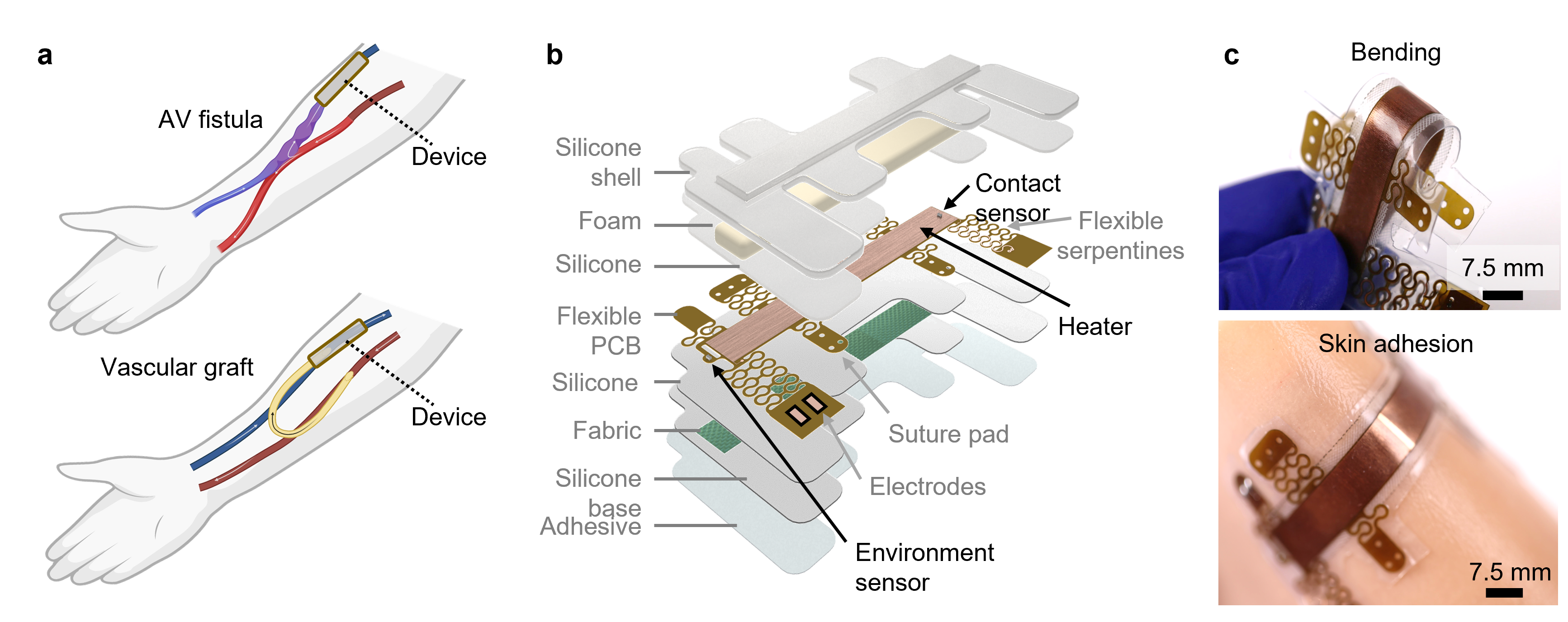
Bridging Clinical Gaps
The idea for this project emerged from conversations with interventional radiologists and bioengineers. Technologies for measuring biofluid flow rates had been in development in the Rogers Lab for several years, but they were all directed toward low flow rates (≤10 mL/min) using calorimetry5-9. Despite the promise of these technologies, they were not suited for the high flow rates seen in vascular access for hemodialysis.
Frustration with the limitations of existing VA monitoring tools provided further motivation. While tools like ultrasound and optical sensors exist, they either require specialized expertise or fail to detect critical changes at physiologically relevant flow rates. This highlighted an unmet need for a device that is sensitive, reliable, and accessible for patients to use in their everyday lives.
From Concept to Reality: The Journey
Developing the thermal sensor was an iterative process marked by both triumphs and setbacks. Early designs relied on calorimetry, but we encountered significant challenges with sensitivity at higher flow rates—common in healthy VAs. Transitioning to anemometric principles allowed us to overcome these limitations. By engineering a modular, flexible device that combines heating elements with precision temperature sensors, we achieved a monotonic response across a broad range of flows.
Bench testing in vascular access models and simulations using finite element analysis (FEA) validated the device's performance. Preclinical studies in swine and on-body measurements demonstrated its sensitivity and real-time responsiveness. Observing the device detect gradual stenosis or rapid onset thrombosis with near-immediate feedback during these trials was a watershed moment.
However, translating lab success into clinical utility is no small feat. One of our most significant hurdles is ensuring the device can function reliably on human tissue—a complex, variable substrate. Iterative testing with biomimetic models and real tissue allowed us to refine the device design, ensuring it adhered conformally and accounted for variations like skin thickness and hydration.
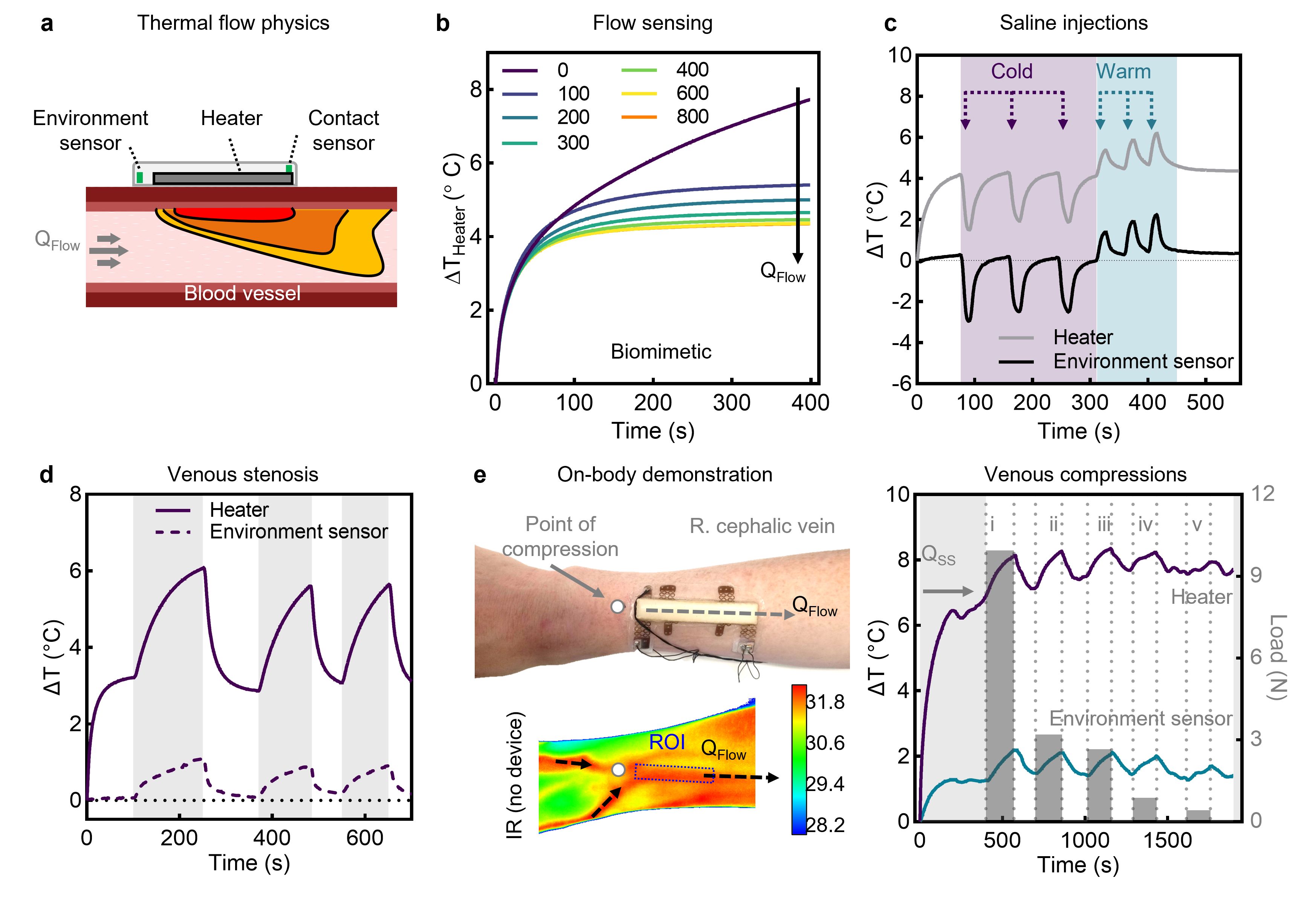
Challenges and Breakthroughs
The journey to develop this sensor was marked by overcoming several critical challenges. Thermal stability was an initial hurdle, as environmental fluctuations introduced noise into the measurements. By incorporating an insulating layer and calibrating for ambient temperature changes, we mitigated these effects, ensuring reliable performance in various conditions.
Achieving sensitivity at both low and high flow rates required careful optimization of the heater geometry and sensor placement. An elongated heater design proved crucial for directing heat effectively and maximizing sensitivity to flow variations. Finally, ensuring biocompatibility was paramount. Extensive testing demonstrated that the device operates safely without causing discomfort or harm to the skin and underlying tissues, adhering to rigorous safety standards.
Real-Life Testing and Future Implications
One of the most rewarding aspects of this work has been witnessing the potential real-world applications of the device. In preclinical studies, the sensor demonstrated the ability to detect the earliest signs of stenosis and thrombosis, enabling immediate intervention. This capability could drastically improve patient outcomes by reducing the need for emergency procedures and preventing the total failure of vascular access.
The potential applications of this technology extend beyond dialysis care. It could be adapted for early detection of vascular conditions in patients with diabetes or cardiovascular diseases. Its modular design also makes it a promising candidate for integration into other wearable health monitoring platforms, paving the way for broader clinical use.
While the current prototype demonstrates immense potential, several advancements are needed to refine the technology further. Incorporating wireless connectivity, such as Bluetooth, will enable seamless at-home monitoring by allowing continuous data transmission to smartphones or clinical systems. Miniaturizing the device and improving its flexibility will enhance comfort for long-term wear, making it more practical for everyday use. Additionally, future versions will feature smart feedback systems capable of adapting measurement protocols in real-time, improving both accuracy and power efficiency.
This technology could revolutionize the standard of care for dialysis patients, empowering them to take a proactive role in their health while reducing the burden on healthcare systems. Beyond dialysis, it holds promise for broader applications, such as detecting vascular conditions in patients with diabetes or cardiovascular diseases. Its modular design makes it a strong candidate for integration into other wearable health monitoring platforms. As this technology evolves, we look forward to its potential to transform patient care in meaningful ways.
References
1 Perlman, R. L. et al. Quality of life in chronic kidney disease (ckd): A cross-sectional analysis in the renal research institute-ckd study. American Journal of Kidney Diseases 45, 658-666 (2005). https://doi.org:https://doi.org/10.1053/j.ajkd.2004.12.021
2 Pockros, B. M., Finch, D. J. & Weiner, D. E. Dialysis and total health care costs in the united states and worldwide: The financial impact of a single-payer dominant system in the us. Journal of the American Society of Nephrology 32, 2137-2139 (2021). https://doi.org:10.1681/asn.2021010082
3 Oliver, M. J. The science of fistula maturation. J Am Soc Nephrol 29, 2607-2609 (2018). https://doi.org:10.1681/asn.2018090922
4 Madhvapathy, S. R. et al. Advanced thermal sensing techniques for characterizing the physical properties of skin. Applied Physics Reviews 9, 041307 (2022). https://doi.org:10.1063/5.0095157
5 Krishnan, S. R. et al. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. npj Digital Medicine 3, 29 (2020). https://doi.org:10.1038/s41746-020-0239-1
6 Lu, D. et al. Implantable, wireless, self-fixing thermal sensors for continuous measurements of microvascular blood flow in flaps and organ grafts. Biosensors and Bioelectronics 206, 114145 (2022). https://doi.org:https://doi.org/10.1016/j.bios.2022.114145
7 Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nature Electronics 4, 302-312 (2021). https://doi.org:10.1038/s41928-021-00556-2
8 Webb, R. C. et al. Epidermal devices for noninvasive, precise, and continuous mapping of macrovascular and microvascular blood flow. Sci Adv 1, e1500701 (2015). https://doi.org:10.1126/sciadv.1500701
9 Krishnan, S. R. et al. Epidermal electronics for noninvasive, wireless, quantitative assessment of ventricular shunt function in patients with hydrocephalus. Science Translational Medicine 10 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in