The bigger picture
The second law of thermodynamics determines the behavior of matter. Generally understood within the context of equilibrium, it suggests that a closed system, devoid of energy input, will ultimately evolve toward its most thermodynamically stable state. In contrast, living organisms function as dissipative structures, continuously absorbing and converting energy through their interactions with the environment. This allows them to maintain an intrinsic order even amid seemingly chaotic conditions. Inspired by biological self-organization, the creation of far-from-equilibrium chemical entities that “consume” free energy will offer valuable insights into the essence of life and contribute to the advancement of active matter.
Dissipative assembled active droplets drive Marangoni flow
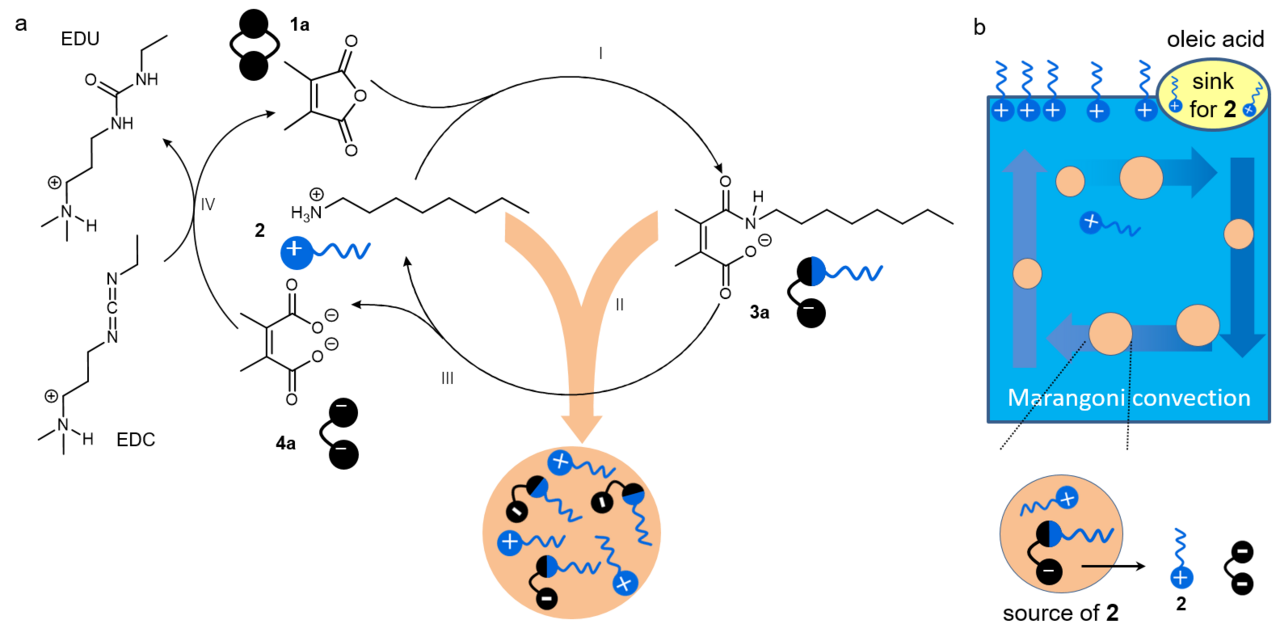
Dissipative amide chemistry
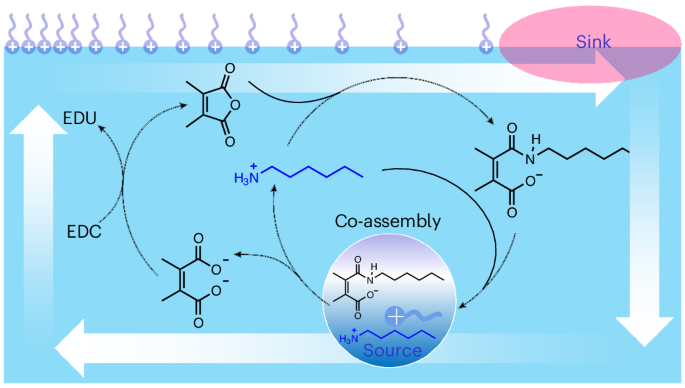
Our study began with establishing new forms of dissipative chemistry, drawing upon principles of classical organic chemistry. One well-known reaction is the condensation of amines with anhydrides to form amides in organic solvents1. Furthermore, under acidic conditions, some amides can undergo spontaneous hydrolysis due to the presence of a neighboring carboxylic-acid group, which catalyzes the hydrolytic reaction intramolecularly2. We tried to integrate the two reactions into a single system. Specifically, by mixing octylamine (2) with maleic anhydride (1a) in an aqueous solution, both the formation and cleavage of the amide bond in (3a) occur simultaneously but via distinct reaction pathways, thereby generating a dissipative reaction network. Additionally, the incorporation of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a secondary fuel molecule allows the hydrolyzed diacid waste (4a) to regenerate amides with 2, thereby sustaining a continuous dissipative cycle.
Dissipative self-assembly of droplets
Droplets form as a result of a chemical reaction between compounds 1a and 2, followed by the co-assembly of the amide product (3a) and 2 based on intermolecular electrostatic and hydrophobic interactions. The hydrophobic regions within these droplets facilitate the dissolution of maleic anhydride, which accelerates the reaction and enables autopoiesis. By modulating the supply of chemical fuels, the growth dynamics of the droplets can be precisely controlled. An increased addition of chemical fuels leads to a double-pulse growth pattern: the turbidity of the mixture containing 2 and 1a initially increases, then decreases sharply, followed by another increase before ultimately vanishing. This behavior arises from the interplay between the dissipative reaction and the co-assembly process, mediated by the antagonistic effects on the concentrations of 3a and 2. Moreover, when the droplets are fully depleted and disappear, reintroducing chemical fuels allows for their regeneration, highlighting the transient nature of the structures.
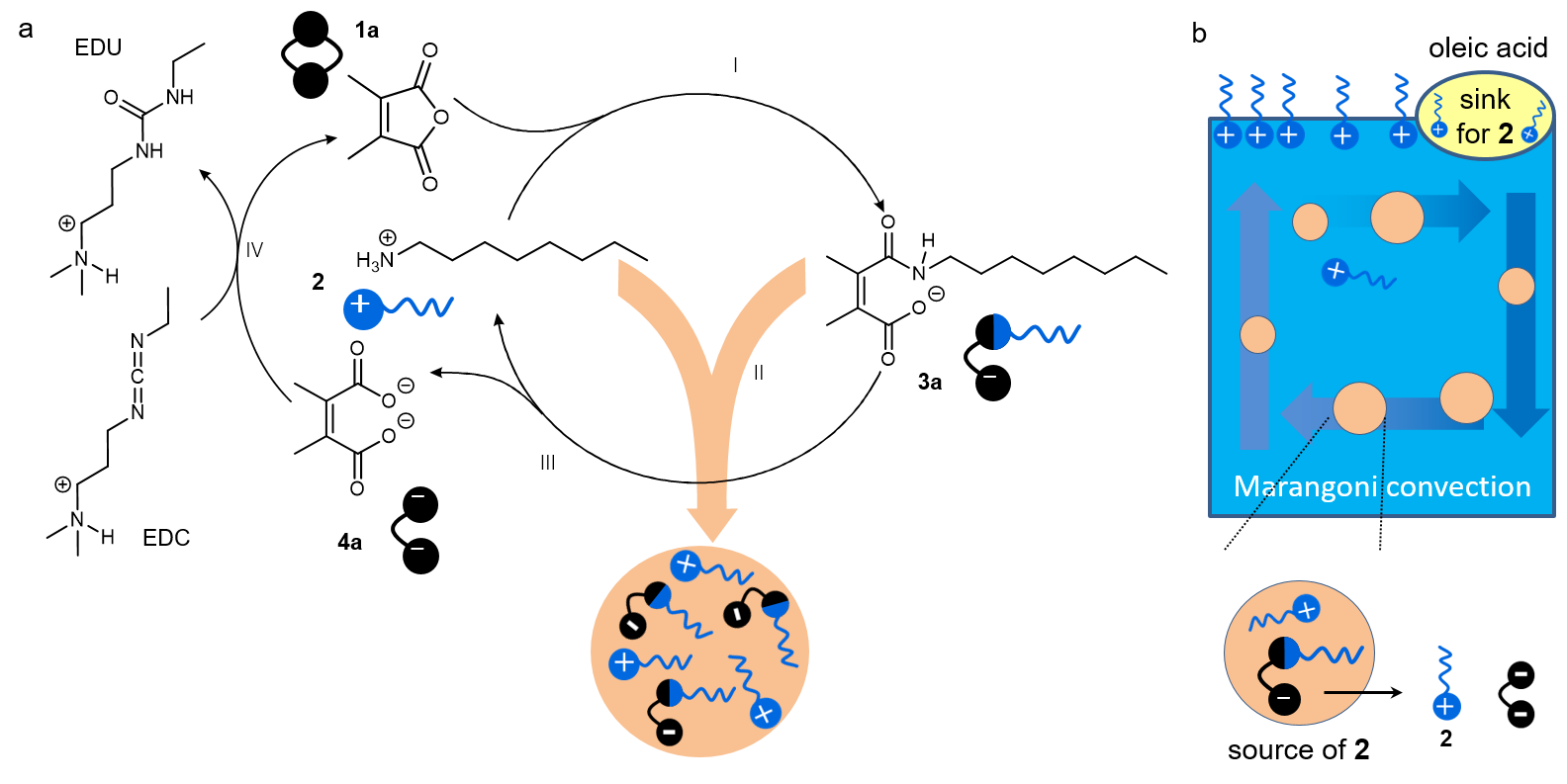
Active droplets as energy converter
With dissipative structures at our disposal, we were eager to explore their unique properties. While theoretical analysis suggests the existence of high-energy states in dissipative self-assembly, directly capturing these structures experimentally has proven challenging3. There wass no clear indication of what to do next. We decided to add oleic acid to the droplet solution in an effort to diversify the components of the system, following a strategy employed in other studies4. An unexpected phenomenon was observed, where the droplets migrated toward the oleic acid at the air-water interface. Moreover, the rate and duration of their movement could be controlled by varying the amount of chemical fuels added. Initially puzzled, we performed theoretical calculations that support a source-sink model: hydrolyzed droplets release octylamine, which is then absorbed by the oleic acid, creating a concentration gradient. This gradient induces variations in surface tension, triggering the Marangoni effect, which drives fluid movement from areas of lower surface tension to those of higher surface tension, thereby transporting the droplets. In this way, the free energy stored in the amide bonds is captured and utilized by the dissipative active droplets, converting it into mechanical energy to perform work, rather than thermal dissipation.
Outlook
In this system, the chemical fuels drive the synthesis of amide bonds at the molecular level, promote the formation of high-energy active droplets at the nanoscale, and facilitate fluid motion to enable droplet movement at the macroscopic scale. This multi-scale energy conversion is based on the hierarchical organization of two kinds of dissipative structures (i.e. active droplets and Marangoni flow). The work highlights the potential of mechanical functions within dissipative assemblies, which may pave the way for the development of novel motors and soft robots. Moreover, chemical control of the Marangoni effect can enhance the precision of material transport and offer promising avenues for developing complex flow patterns. Notably, the droplet system, composed of simple components, can serve as a primitive cell model capable of chemotactic movement, potentially leading to more intricate collective behaviors. Without a doubt, dissipative chemistry will expand our physicochemical understanding, providing deeper insights into the nature of complexity. Exciting new properties and functions await discovery!
References:
1. Matysiak, B. M., Monreal Santiago, G. & Otto, S. Teaching an Old Compound New Tricks: Reversible Transamidation in Maleamic Acids. Chem. Eur. J. 28, e202201043 (2022).
2. Kirby, A. J. Effective molarities for intramolecular reactions. Adv. Phys. Org. Chem. 17, 183–278 (1980).
3. Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotechnol. 13, 882–889 (2018).
4. Meredith, C. H. et al. Predator–prey interactions between droplets driven by non-reciprocal oil exchange. Nat. Chem. 12, 1136–1142 (2020).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in