Integrating a New Template for Building Adjustable Mechanically Interlocked Molecules into the Toolbox
Published in Chemistry
The pursuit of designing and synthesizing complex molecular architectures continuously fuels innovation and discovery. Our latest publication, "Conjugated Bis(enaminones) as Effective Templates for Rotaxane Assembly and Their Post-Synthetic Modifications",[1] explores the synthesis of mechanically interlocked molecules (MIMs), focusing on hydrogen-bonded amide-based rotaxanes and the preparation of other rotaxanes preserving the integrity of their mechanical bond. Classically, these rotaxanes have been assembled using a five-component, [2+2+1] clipping methodology, employing different templates with yields ranging from 8% to 70%. Notably, using fumaramides as templates has proven highly effective, achieving isolated yields of up to 97%.[2] When designing a programmable MIM requires a low-yielding template, constructing an intermediate system can be advantageous. This intermediate can undergo post-synthetic modifications,[3] allowing a further structural transformation of the interlocked architecture after initial assembly.
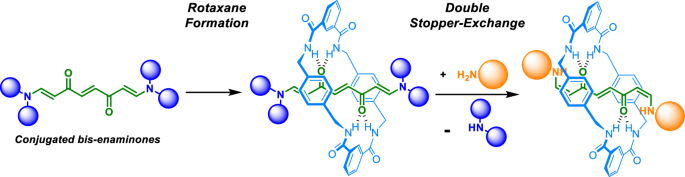
In this investigation, our team shows that conjugated bis(enaminones) are effective templates for assembling hydrogen-bonded amide-based rotaxanes. We hypothesize that enhancing the hydrogen-bonding acceptor ability of the oxygen atoms in the carbonyl groups of the template could increase rotaxane yield. Incorporating a modest double bond between the carbonyl carbon and the nitrogen atoms of the amide function is a reasonable approach (and computationally supported) to achieve this goal and provide access to functional groups capable of further structural modifications. Therefore, Dr. Razi prepared a series of conjugated bis(enaminone)-based threads and assessed their ability to template rotaxane formation. The five-component clipping reactions involving these threads and appropriate stoppers demonstrated full conversion without any unreacted starting material. For instance, the bis(enaminone)-based thread 3a, featuring four benzyl groups as stoppers, resulted in a 90% yield of rotaxane 4a (Fig. 1), significantly higher than the 36% yield obtained from its fumaramide-based analogue.
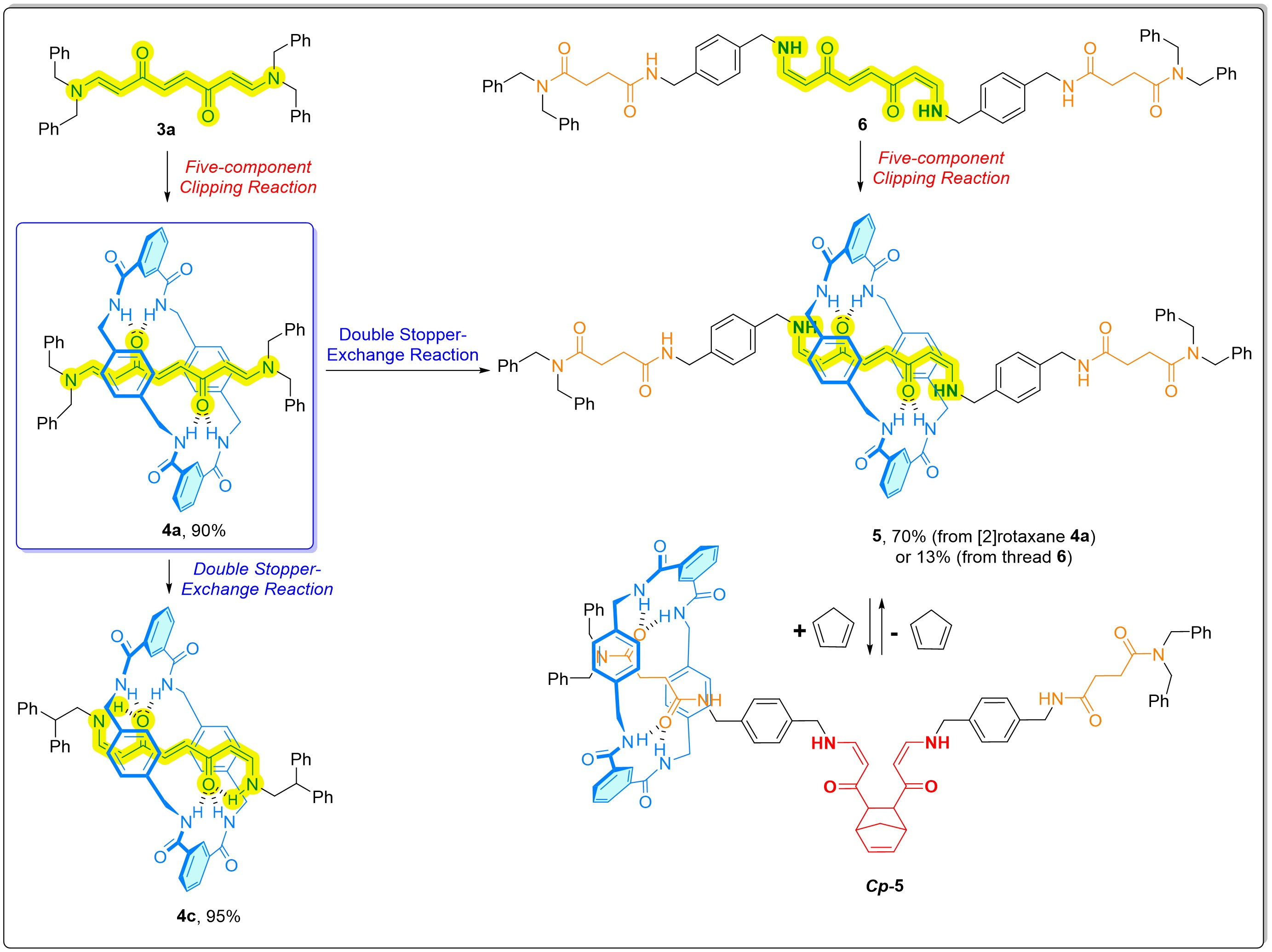
Fig. 1. Synthesis of amide-based rotaxanes by clipping methodology and via double stopper-exchange reaction. Reversible control of ring position in shuttle 5. Conjugated bis(enaminones) moieties are highlighted in yellow. See experimental details in the published article.
To further understand the structural properties and internal dynamics of these novel rotaxanes, we carried out single-crystal X-ray diffraction and variable-temperature NMR experiments. The SCXRD analysis of rotaxane 4a revealed two intramolecular hydrogen bonds between the thread and the macrocycle instead of the four intercomponent hydrogen bonds observed in the fumaramide surrogate. Interestingly, in solution, we observed differences in the rotational dynamics of the macrocycle, with the bis(enaminone)-based system exhibiting weaker interactions and lower energy barriers for rotation compared to the fumaramide-based system.
A key feature of this work is the development of a novel double stopper-exchange protocol. This post-synthetic modification allows for the conversion of rotaxane 4a into various interlocked derivatives with new stoppers while preserving the mechanical bond. For example, reaction with 2,2-diphenylethylamine resulted in a 95% yield of rotaxane 4c (Fig. 1). This approach not only expands the versatility and usefulness of the synthesized rotaxanes but also facilitates the straightforward preparation of more elaborated interlocked systems such as the molecular shuttle 5, circumventing challenges associated with traditional clipping methodology (in this case, 5 was obtained in only 13% yield via clipping). To control the position of the ring in 5 (Fig. 1), we employed a chemically induced reversible process via a cycloaddition/retrocycloaddition sequence.[4,5]
Our research[1] showcases the potential of conjugated bis(enaminones) as templates for rotaxane synthesis and their subsequent modifications. Efficiently synthesizing rotaxanes with high yields and controlling their internal dynamics opens new avenues for designing sophisticated molecular architectures. As we continue to explore the applications of these interlocked compounds, we anticipate further advances in developing molecular machines and devices, stay tuned for more updates.
[1] Razi, S.S., Marin-Luna, M., Alajarin, M., Martinez-Cuezva, A., Berna, J. Conjugated bis(enaminones) as effective templates for rotaxane assembly and their post-synthetic modifications. Commun. Chem. 7, 170 (2024).
[2] Gatti, F. G., Leigh, D. A., Nepogodiev, S. A., Slawin, A. M. Z., Teat, S. J. & Wong, J. K. Y. Stiff, and Sticky in the Right Places: The Dramatic Influence of Preorganizing Guest Binding Sites on the Hydrogen Bond-Directed Assembly of Rotaxanes. J. Am. Chem. Soc. 123, 5983-5989 (2001).
[3] Waelès, P., Gauthier, M. & Coutrot, F. Challenges and Opportunities in the Post-Synthetic Modification of Interlocked Molecules. Angew. Chem. Int. Ed. 60, 16778-16799 (2021).
[4] Leigh, D. A. & Pérez, E. M. Shuttling through reversible covalent chemistry. Chem. Commun. 2262–2263 (2004).
[5] Saura-Sanmartin, A., Nicolas-Garcia, T., Pastor, A., Quiñonero, D., Alajarin, M., Martinez-Cuezva, A. & Berna, J. Control of the assembly of a cyclic hetero[4]pseudorotaxane from a self-complementary [2]rotaxane. Chem. Sci. 14, 4143–4151 (2023).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in