Adeno-associated virus-mediated trastuzumab delivery to the central nervous system for human epidermal growth factor receptor 2+ brain metastasis
Published in General & Internal Medicine
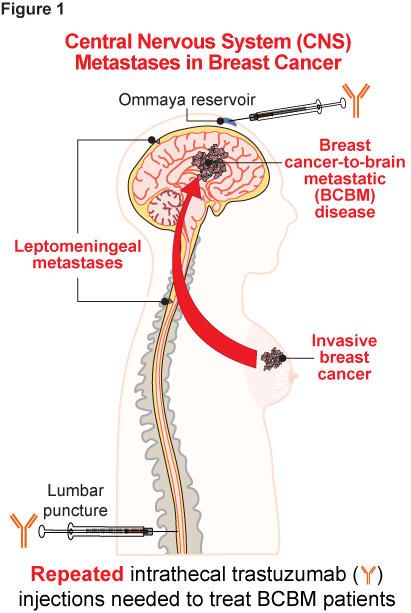
Breast cancer represents a potentially devastating diagnosis. Human epidermal growth factor receptor 2 (HER2) overexpression/amplification occurs in 15-20% of breast cancers and is associated with an aggressive phenotype; up to half of patients with metastatic HER2+ breast cancer will develop central nervous system (CNS) metastases (1). Aside from morbidity and mortality, metastatic breast cancer is associated with serious disruption to the patient and their caregivers’ overall quality of life and employment (2, 3); it can also carry a significant economic burden (4). While trastuzumab (Herceptin®), a humanized anti-HER2 IgG1 monoclonal antibody, revolutionized HER2+ breast cancer treatment since its approval in 1998 (5), sustaining an effective drug response in patients with HER2+ breast cancer-to-brain (BCBM) and/or leptomeningeal metastases (Figure 1) - a rare but catastrophic breast cancer complication (6) - requires repeated (weekly) intrathecal (IT) trastuzumab injections. Frequent IT dosing is associated with an increased risk of post-procedural complications (e.g., bleeding and infections), and it also requires access to specialized healthcare facilities, which may not be always feasible or even affordable (7-9). Given these constraints, we developed a single-dose gene therapy approach comprising a recombinant adeno-associated virus (AAV) vector engineered to constitutively express trastuzumab within the CNS.
Recombinant AAV vectors are replication-deficient virus vectors that utilize cellular machinery to produce the encoded therapeutic protein. Our group has shown that intrathecal delivery of neurotropic AAV vectors results in widespread transduction and transgene expression in the brain and spinal cord of rodents and large animals (dogs and nonhuman primates [NHPs]) (10-12). In collaboration with the University of Pennsylvania’s Division of Neuroradiology (Dr. Bryan Pukenas), we have developed an intra-cisterna magna (ICM) technique to deliver AAV using a computed tomography fluoroscopy protocol (13). Over 2,000 ICM injections in 485 primates have been safely conducted, with only four adverse events. Having demonstrated this technique’s safety and feasibility, several first-in-human trials are underway using ICM delivery of recombinant AAV vectors to treat neurodegenerative diseases (e.g., gangliosidosis [NCT04713475], early infantile Krabbe disease [NCT04771416], and frontotemporal dementia [NCT04747431]).
We designed an AAV vector that expresses a codon-optimized, ubiquitin C (UbC)-promoter-driven trastuzumab sequence (AAV9.UbC.trastuzumab). Transgene expression was evaluated in mice and NHPs using real-time PCR, ELISA, Western blot, in situ hybridization, single-nucleus RNA sequencing, and liquid chromatography-mass spectrometry (Figure 2). We first quantified trastuzumab levels in the serum and brain parenchyma of Rag1 KO mice 4 weeks after ICV delivery of AAV9.UbC.trastuzumab vector and detected robust systemic and local transgene expression. We then analyzed expression in adult female Indian rhesus macaques with pre-existing AAV9-neutralizing antibodies (titer range ≥1:10–1:40) following ICM administration. The treatment was well tolerated (36–37 days in-life) and resulted in transgene expression in CNS tissues and cerebrospinal fluid. Given this, we anticipate any patient with HER2+ CNS metastasis could be treated using this approach, regardless of their anti-AAV antibody titer status, via ICM administration of AAV9 into the CNS.
We next evaluated the anti-tumor efficacy of AAV9.UbC.trastuzumab ICV administration in aggressive cell line-derived xenograft mouse models of HER2+ BCBM in which BT-474 and MDA-MB-453 cell lines were engrafted to the right frontal lobe using a guide-screw system (14). Treatment with AAV9.UbC.trastuzumab delayed tumor progression and extended overall survival compared to control. Lower trastuzumab expression levels were observed in the NHP brain parenchyma than in the tumor challenge experiments in mice, so we investigated whether this lower trastuzumab dose exhibited sufficient anti-tumor activity. A dose-down experiment was conducted to achieve expression levels in xenograft-bearing Rag1 KO mice equivalent to those reached in NHPs. ICV administration of the appropriate AAV9.UbC.trastuzumab dose resulted in a 100% response rate, with all mice in complete remission beyond 12 weeks after engraftment compared with control animals. These promising results highlight how the lower dose achieved in NHP CNS exhibits robust anti-tumor efficacy and reinforce the importance of bridging gaps between translational models during preclinical development.
With AAV9’s proven clinical safety record, these preclinical data indicate that AAV-mediated expression of trastuzumab within the CNS may represent a viable strategy to treat and prevent the emergence of HER2+ CNS metastases in breast cancer patients. Moreover, an effective single dose treatment strategy could help to alleviate the stress and emotional toll that frequent procedures such as IT therapies can have among cancer patients and their caregivers.

References
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-72.
- Williams CP, Gallagher KD, Deehr K, Aswani MS, Azuero A, Daniel CL, et al. Quantifying treatment preferences and their association with financial toxicity in women with breast cancer. Cancer. 2021;127(3):449-57.
- Xiang E, Guzman P, Mims M, Badr H. Balancing Work and Cancer Care: Challenges Faced by Employed Informal Caregivers. Cancers (Basel). 2022;14(17).
- Mahtani R, Oestreicher N, Lalla D, Ogbonnaya A, Saundankar V, Willey J, et al. Health Care Resource Utilization and Costs for Metastatic Breast Cancer Patients Newly Treated with Human Epidermal Growth Factor Receptor 2 (HER2)-Targeted Agents. Clin Breast Cancer. 2022;22(4):e488-e96.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-83.
- Mills MN, King W, Soyano A, Pina Y, Czerniecki BJ, Forsyth PA, et al. Evolving management of HER2+ breast cancer brain metastases and leptomeningeal disease. J Neurooncol. 2022;157(2):249-69.
- Bousquet G, Darrouzain F, de Bazelaire C, Ternant D, Barranger E, Winterman S, et al. Intrathecal Trastuzumab Halts Progression of CNS Metastases in Breast Cancer. J Clin Oncol. 2016;34(16):e151-5.
- Bonneau C, Paintaud G, Tredan O, Dubot C, Desvignes C, Dieras V, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75-84.
- Delhaas EM, Huygen F. Complications associated with intrathecal drug delivery systems. BJA Educ. 2020;20(2):51-7.
- Hinderer C, Katz N, Dyer C, Goode T, Johansson J, Bell P, et al. Translational Feasibility of Lumbar Puncture for Intrathecal AAV Administration. Mol Ther Methods Clin Dev. 2020;17:969-74.
- Hordeaux J, Hinderer C, Goode T, Katz N, Buza EL, Bell P, et al. Toxicology Study of Intra-Cisterna Magna Adeno-Associated Virus 9 Expressing Human Alpha-L-Iduronidase in Rhesus Macaques. Mol Ther Methods Clin Dev. 2018;10:79-88.
- Hordeaux J, Jeffrey BA, Jian J, Choudhury GR, Michalson K, Mitchell TW, et al. Efficacy and Safety of a Krabbe Disease Gene Therapy. Hum Gene Ther. 2022;33(9-10):499-517.
- Hinderer C, Bell P, Vite CH, Louboutin JP, Grant R, Bote E, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev. 2014;1:14051.
- Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92(2):326-33.
Follow the Topic
-
Cancer Gene Therapy

The essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in