Advancing Antibody Conjugates with Ubi-Tagging
Published in Bioengineering & Biotechnology and Biomedical Research
What is Ubi-Tagging?
Ubi-tagging employs the small protein ubiquitin as a conjugation tag, enabling fast and efficient attachment of various molecular cargo, such as peptides, nanobodies, and even small molecules like fluorescent dyes. This method uses ubiquitination enzymes to achieve site-specific conjugation in as little as 30 minutes, with an impressive average efficiency of 93–96%. The platform’s modularity and precision facilitate the generation of multimeric antibody conjugates, including complex formats like bi-specific T cell engagers. The use of synthetic ubiquitin derivatives further expands ubi-tagging’s potential, enabling chemical modifications for a wide array of applications.
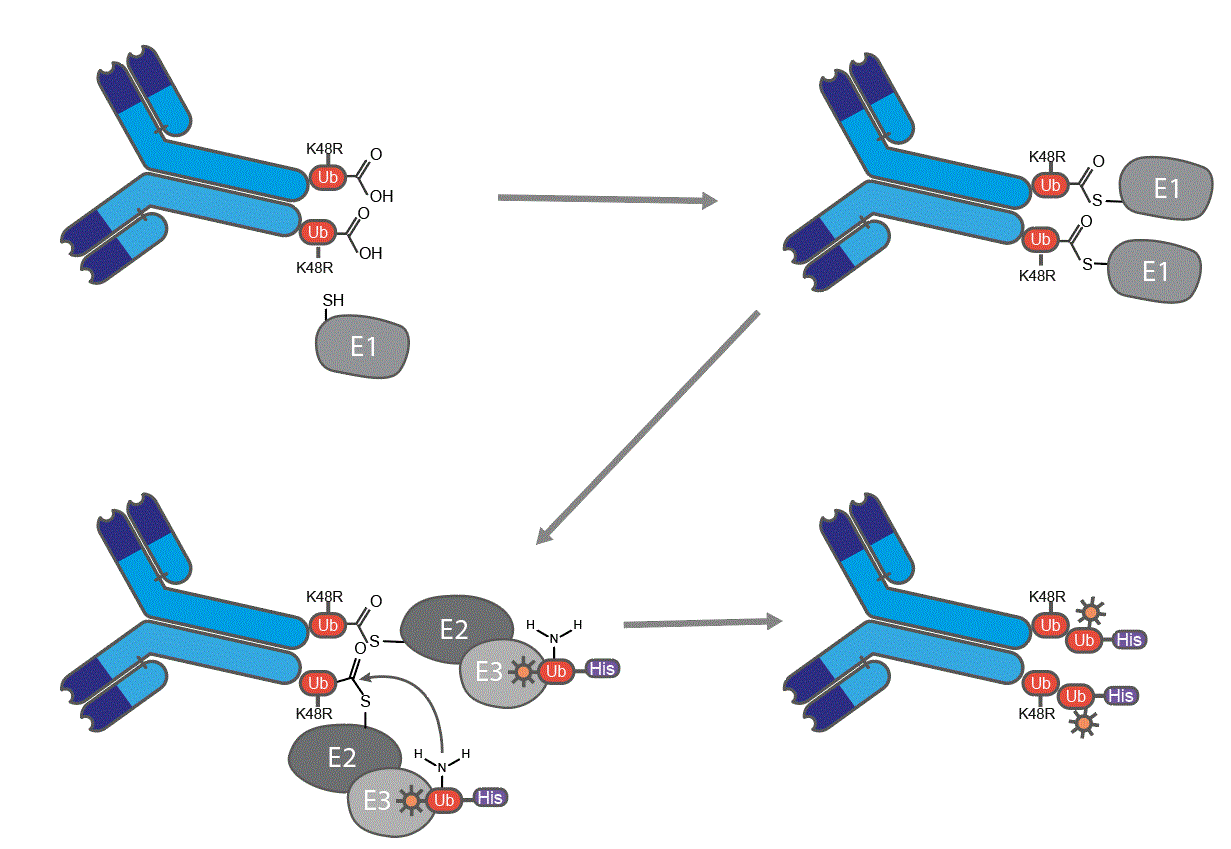

Figure 1: Schematic representation of site-specific heterodimerization of antibody using ubi-tagging. The antibody has in the fused ubiquitin the lysine (K) 48 mutated into an arginine, to prevent homodimer formation and polymerization. The acceptor ubiquitin carries the corresponding conjugation lysine residue (K48) while having an unreactive C‑terminus by blocking the C-terminus with a His-tag.
Key Findings
-
Efficiency and Specificity:
Ubi-tagging consistently delivers homogenous products, demonstrating robust efficiency across a range of proteins, from small nanobodies to full-sized monoclonal antibodies.
-
Multimeric Conjugates:
The platform enables precise assembly of multi-valent and hetero-multimeric antibody conjugates, offering unprecedented control over product configuration.
-
Improved Antigen Delivery:
Using ubi-tagging for dendritic cell-targeted antigen delivery led to enhanced T cell activation in vivo, outperforming state-of-the-art methods like sortagging.
-
Enhanced Solubility:
Ubi-tagging improved the solubility of challenging nanobody-antigen conjugates, reducing aggregation and increasing functional efficacy.
-
Broad Compatibility:
The platform integrates recombinant and chemically synthesized ubiquitin, accommodating diverse modifications and expanding its versatility for preclinical, diagnostic, and therapeutic uses.
Challenges and Insights
Despite its promise, ubi-tagging does have limitations. The ubiquitin tag is relatively larger than peptide tags used in other conjugation methods, which could constrain its use in specific applications. Additionally, the system relies on recombinant ubiquitination enzymes, requiring production and storage considerations.
However, the robust chemical synthesis of ubiquitin derivatives and the availability of various linkage-specific ubiquitination enzymes open the door to further innovation. These tools allow for the creation of highly complex conjugates with predefined functionalities.
The Bigger Picture: Advancing Therapeutics
In our study, we demonstrated the potential of ubi-tagging in antigen delivery, where dendritic cell-targeted vaccines showed superior T cell activation and selective on-target uptake in the spleen. This efficiency may stem from enhanced solubility, reduced aggregation, and unique intracellular processing pathways influenced by ubiquitin’s natural signaling roles.
These findings suggest ubi-tagging could play a pivotal role in the development of next-generation antibody-based therapies, particularly in fields like cancer immunotherapy and vaccine design.
Looking Forward
Our work highlights the transformative potential of ubi-tagging as a fast, efficient, and modular conjugation platform. Moving forward, we plan to explore its applications in multimeric antibody conjugates from an immune-oncology and auto-immune perspective.
By enabling the creation of precisely engineered conjugates, ubi-tagging has the potential to address current limitations in antibody engineering and pave the way for groundbreaking advancements in medicine. We hope this platform will inspire researchers to explore its possibilities and collaborate in pushing the boundaries of antibody conjugation.
Teamwork
My good friend Huib Ovaa, a trained organic chemist, invited me to one of his lab meetings. His laboratory was renowned worldwide for its pioneering research into the complexities of protein ubiquitylation and its implications for health and disease. Employing advanced chemical tools and innovative chemical-biological strategies, the lab tackled difficult and interesting biological questions. During one such meeting, the team faced challenges in producing recombinant antibodies. Having recently published a method to genetically modify hybridomas in order to obtain modified antibodies1, I shared my insights. By the end of that meeting, an idea emerged: could the ubiquitin system be repurposed as a conjugation platform for recombinant proteins? This marked the beginning of our journey. Together with Angela el Hebieshy, a talented PhD student in the Ovaa lab, we set out to investigate whether ubiquitin could serve as a fusion tag for site-specific and controlled antibody conjugation. In the spring of 2020, the more or less unexpected passing of Huib Ovaa left an immense void. Progress on the project slowed as we navigated the loss of Huib, significant budget cuts at the institute and the COVID-19 pandemic. Yet, resilience is the foundation of science. After discussions with Martijn Verdoes and his PhD student, Zach Wijfjes, they joined this project. Martijn's expertise as a chemical-immunologist specializing in dendritic cell vaccinations propelled the project forward, taking it to new horizon of targeted vaccinations and beyond. This work stands as a testament to the power of collaboration, innovation, and cross-field collaborations.
Angela F. El Hebieshy & Ferenc A. Scheeren
Reference:
1 van der Schoot, J. M. S. et al. Functional diversification of hybridoma-produced antibodies by CRISPR/HDR genomic engineering. Sci Adv 5, eaaw1822, doi:10.1126/sciadv.aaw1822 (2019).
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in