Aggregation-induced electrosynthesis
Published in Chemistry
Reaction design
Water is the greenest solvent for the production of substances and provides an excellent resource for hydrogen production via electrocatalytic reduction. However, owing to the poor solubility of common organic compounds, electrochemical organic synthesis typically requires organic solvents with a supporting electrolyte to ensure the conductivity of the reaction medium, which negatively impacts the strength of electroorganic synthesis. How organic synthesis can adapt to whole aqueous media is an essential scientific question with vista for application.
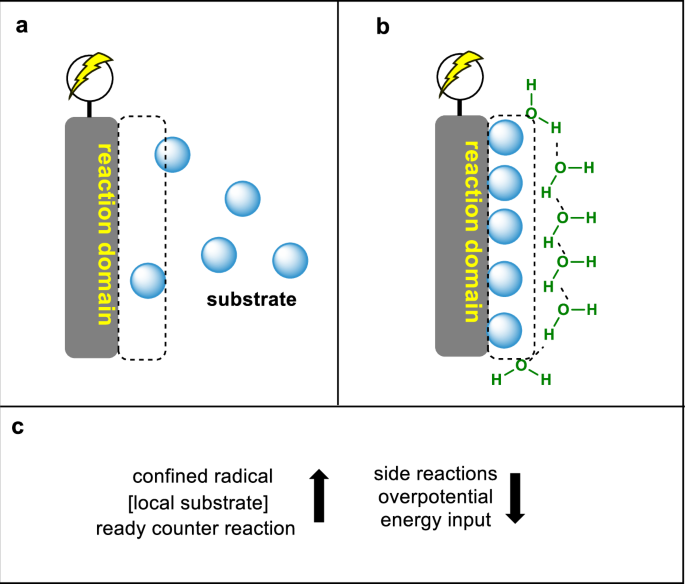
Recently, the Cheng group reported that aggregation-induced electrochemical radical cross coupling of unsaturated compounds was driven by surficial tension in whole aqueous media (Figure 1). A key finding in this work is that aggregation of the substrate due to its solubility is a boost for the reaction instead of an obstacle. In this model, the aggregated substrate converts to a confined radical that cross-couples with a water-soluble radical. The product has medium solubility and diffuses to the aqueous phase, leaving reactive sites at the electrode for the incoming substrate. As the local high concentration of substrate at the electrode was maintained, another significant advantage of aggregation is the high chemoselectivity even at high conversions.
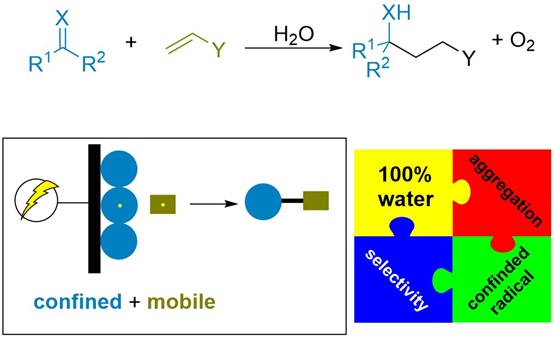
Figure 1. The rationale of aggregation-induced electrosynthesis.
Under this general model, the aldehyde reacts with acrylonitrile, resulting in a cross-coupling product with up to 90% yield in whole water media with a catalytic amount of NaOH as an additive. In the graphite felt cathode, aldehyde clearly aggregated, and acrylonitrile was soluble in water. This multiphase model could convert aldehyde to delta-hydroxyl-nitrile in up to 90% yield. Compared with homogenous reactions, aggregation-induced electrosynthesis results in distinct outcomes. For example, when the reaction was conducted in MeCN, the dimerization of aldehyde and acrylonitrile took place without the observation of cross-coupling products. In other evaluations, even the cosolvent of water/MeCN resulted in inferior yields. Other methods to increase the solubility of the substrate in water also decrease the yield. For example, when the surfactant sodium dodecyl benzene sulfonate was utilized, a significant yield decrease occurred.
Inspired by this discovery, Li conducted quantitative experiments to understand why the aggregation effect is critical for the desired C‒C bond formation. First, mass distribution analysis revealed that more than 90% of the substrate aggregated at the electrode. This effect increased the local concentration of substrate to the limit of condensed matter. In this environment, cathodic reduction to the substrate takes place, generating a radical species. Moreover, the reduction of acrylonitrile yielded another radical species. At the aggregated local site, two radical species exhibited unique behavior. For the aldehyde radical, the concentration increased with increasing local concentration in the aggregated state. However, this radical does not dimerize, as the diffusion of radical species is inhibited. On the other hand, the acrylonitrile-derived radical had a low concentration, as acrylonitrile is present in a homogenous solution. Therefore, the confined radical at high concentrations combined with the mobile radical at low concentrations, resulting in ideal selectivity. The product subsequently has a nitrile group and a new hydroxyl group, both of which benefit solubility and facilitate the diffusion of the product to aqueous media, providing aggregation sites for the incoming substrate. The aggregation dramatically increased the amount of insoluble radicals released from the microseconds to several minutes. In the aggregation state, even the radical clock did not respond, as it was confined. Therefore, this model could be extended to more substrates with poor solubility.
The inspiration from the Reviewers:
The reviewers provided several important suggestions to help elucidate the reaction mechanism. For example, the solubilities of the compounds in this reaction are essential information for determining the reaction design. Li measured the solubilities of different chemicals in water via 1H NMR using an internal standard, confirming the consistency of the solubility gradient with the mass diffusion profile. Another suggestion from the reviewer is that the reaction takes place at the anode. Li used a combination of a coulombmeter, a gas flow meter and an oxygen meter to analyse the total amount of oxygen evolution from the reaction. This experiment established that the OER was a counterreaction in the electrochemical process. This result further demonstrates the advantage of reactions conducted in water, where the side reactions take place in a traceless manner.
For practice, the water is readily separated from the products via cold filtration, and the performance is maintained over the next several cycles. In addition, the reaction is facilitated by the oxygen evolution reaction at the counter electrode, which is highly ready in a water solution.
Related Content
The link to the publication:
Journal: Nature Communications.
DOI : https://doi.org/10.1038/s41467-024-52042-w
Title : Aggregation-induced C–C bond formation on an electrode driven by the surface tension of water
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in