Antibiotics, microbiota-derived peptidoglycan, and Candida albicans infection
Published in Microbiology

In polymicrobial communities in nature, microorganisms often engage a diverse range of small molecules as messengers in conversations with one another to influence and regulate their growth and phenotypes. One of the most densely populated microbial niches on Earth is the human gut, where trillions of bacteria, fungi, and viruses co-inhabit to form the gut microbiota. Alterations of the composition of this community can have significant impacts on host physiology, critically influencing health and disease.
We are interested in the roles of microbial molecules in the inter-kingdom communications in host gut microbiota, particularly in the context of fungal infections caused by Candida albicans. Elucidating and modulating these molecules may offer insights on risk factors and disease determinants, which may lead to innovative strategies for infection prevention and treatment.
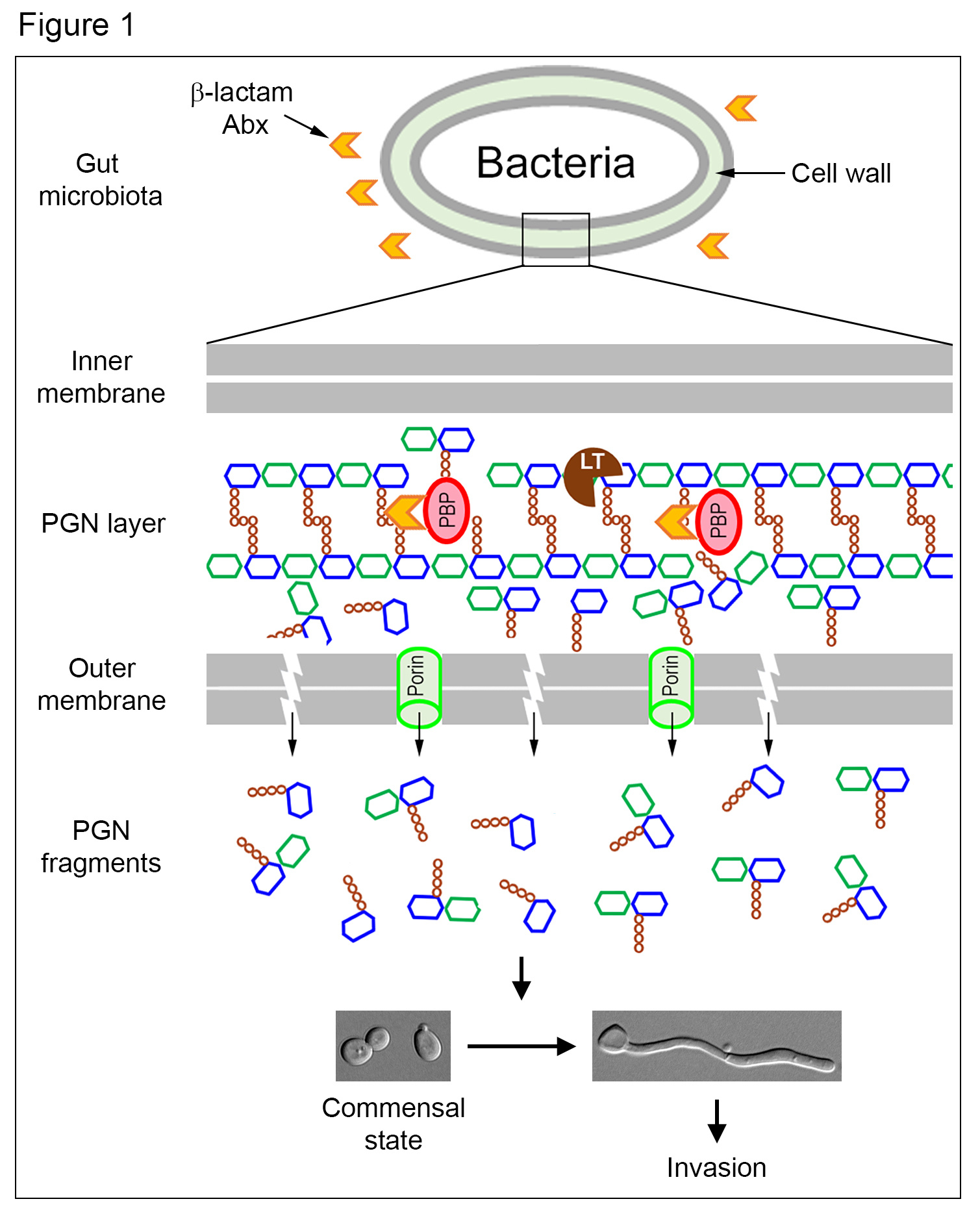
The molecules that we focus on is the peptidoglycan (PGN), a conserved and essential component of the bacterial cell wall. During normal growth and division, bacteria can shed PGN fragments into the surrounding, which are known to act as ancient signalling molecules that influence the development of higher order organisms, such as Hawaiian bobtail squid and the fungus C. albicans (1,2). C. albicans is a harmless commensal in the gut of healthy people. However, it can become a deadly pathogen under certain circumstances, such as immunosuppression, mucosal barrier damage, and the use of antibiotics (Abx). In a previous study, we found that PGN fragments can stimulate the invasive hyphal growth of C. albicans in vitro (2).
An important clinical risk factor for invasive C. albicans infection is the use of Abx. However, the reasons behind it were not clear. A clue emerged from a report that β-lactam Abx causes the malfunctioning of PGN biosynthetic machinery, churning out large quantities of PGN subunits (3), whose structures highly resemble to those we previously identified to be active in triggering C. albicans hyphal growth (2). This enlightening connection led us to hypothesize that treatment with β-lactam Abx would cause the trillions of gut bacteria to release a massive amount of PGN subunits, which could promote C. albicans hyphal growth to penetrate mucosal barriers, leading to tissue invasion (Figure 1).
To test our hypothesis, we first set up a simple ‘patch assay’ on agar plates, where a bacterial patch was grown side-by-side to the C. albicans patch. Upon applying the Abx solution to the bacterial patch, we monitored the hyphal growth of the C. albicans patch under the microscope. We observed that, despite killing bacteria, the application of β-lactam Abx onto the bacterial patch strongly induced hyphal growth of the C. albicans patch. In contrast, Abx of other mechanisms of action had no significant effect. Similarly, treating bacteria with β-lactam Abx in liquid culture also increased the hyphal induction activity of the liquid broth. None of the Abx alone can induce C. albicans hyphal growth.
Encouraged by the initial observations, we then sought to identify the bacterial molecules that promote hyphal growth. As our hypothesis predicted a significant increase of PGN released by bacteria upon β-lactam Abx treatment, we first quantified PGN in β-lactam Abx-treated bacterial cultures by ELISA using a monoclonal antibody (mAb), which specifically recognizes muramyl dipeptide (4), a motif conserved in PGN across all bacteria. Strikingly, we detected 30-40 times more PGN in β-lactam Abx-treated cultures than untreated controls. When we added the anti-PGN mAb to sequester PGN in the culture medium, it completely abolished its hyphal induction activity, confirming that PGN molecules are responsible for causing C. albicans hyphal growth under the conditions described above.
Next, we used HPLC to fractionate the β-lactam Abx-treated culture broth of Staphylococcus aureus. We identified the PGN-containing fractions using both anti-PGN ELISA and hyphal induction assay. Subsequent mass spectrometry analysis revealed that the active fraction indeed contained a signature PGN fragment. To demonstrate this and other natural PGN fragments can trigger C. albicans hyphal growth, we purified a collection of PGN fragments from both E. coli and S. aureus. Several PGN fragments exhibited potent hyphal induction activity. The data strongly supported our hypothesis of β-lactam Abx causing bacteria to release PGN fragments, which promote C. albicans hyphal growth.
Then we went on to test whether treating mice with β-lactam Abx would promote C. albicans hyphal growth in the gut. We fed β-lactam or non-β-lactam Abx to mice, followed by oral inoculation of C. albicans yeast cells. We then examined the morphologies of C. albicans in feces and the cecum. Strikingly, while all C. albicans cells found in feces and the cecum of control mice were in the yeast form, the majority of C. albicans cells collected from the β-lactam Abx-treated mice were long hyphae. In comparison, none-β-lactam Abx had no significant effect on C. albicans morphology. Furthermore, we demonstrated that the hyphal growth caused β-lactam Abx treatment increased the incidence of systemic dissemination of C. albicans in mice.
Is the C. albicans hyphal growth in the gut following β-lactam Abx treatment due to gut microbiota-derived PGN molecules? To answer this question, we quantified the levels of PGNs in the cecum and the feces at intervals after β-lactam Abx treatment. We detected a sharp increase of PGN within six hours. Intriguingly, the PGN levels remained high for several days. Among several possible explanations, the most likely one is that resistant bacteria continue to grow and release high levels of PGN subunits in the presence of β-lactam Abx.
This study provides a mechanistic explanation for the increased incidences of invasive C. albicans infection following Abx treatment, a long-recognized but poorly understood risk factor. In summary, β-lactam Abx treatment forces the trillions of gut bacteria to produce a large amount of PGN fragments, which transforms the gut environment from suppressing to promoting C. albicans hyphal growth leading to invasion and dissemination (Figure 1). Understanding this mechanism may inform clinicians on the careful choice of antibiotics to minimize C. albicans infection in the future. It also offers new strategies to prevent or reduce the infection by sequestering the gut PGN or inhibiting its production.
http://dx.doi.org/10.1038/s41467-021-22845-2
References
(1) Nyholm, S. V. & McFall-Ngai, M. J. (2004) The winnowing: establishing the squid– vibrio symbiosis. Nature Rev Microbiol 2, 632.
(2) XL Xu, X.L., Lee, R.T.H., Fang, H.M., Wang, Y.M., Li, R., Zou, H, Zhu, Y. & Wang, Y. (2008) Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4, 28.
(3) Cho, H., Uehara, T. & Bernhardt, T. G. (2014) Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159, 1300.
(4) Huang, Z., Wang, J, Xu, X., Wang, H., Qiao, Y., Chu, W.C., Xu, S., Chai, L., Cottier, F., Pavelka, N., Oosting, M., Joosten, L.A.B., Netea, M., Ng, C.Y.L., Leong, K.P., Kundu, P., Lam, K.P., Wang, Y. (2019) Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat Microbiol 4:766.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in