Candida albicans Ssy1 as the extraceullar sensor of gut microbiota-derived peptidoglycan fragments (PGNs)
Published in Microbiology and Biomedical Research
While gut bacteria have long dominated microbiome research, much less attention has been given to the fungal inhabitants of our gut. Among them, Candida albicans stands out as a prevalent commensal fungus found in the oral cavity, gastrointestinal tract, and genitourinary tract, where it coexists with trillions of bacteria. Though typically harmless, C. albicans can become a deadly pathogen, especially in immunocompromised individuals, by switching from a benign yeast form to an invasive hyphal form capable of penetrating tissue and spreading systemically.
But what triggers this transformation?
One key factor is the influence of the gut environmental signals. In particular, peptidoglycan fragments (PGNs) derived from the bacterial cell wall have emerged as potent inducers of hyphal morphogenesis. Under normal conditions, bacteria secrete a substantial amount of PGNs during their growth and division. However, oral intake of β-lactam antibiotics can cause a dramatic surge in PGN levels in the host gut, a phenomenon we call the “PGN-storm”. This PGN-storm strongly promotes C. albicans filamentation and systemic spread in the mouse gut, offering a molecular explanation for why Candida infections often follow long-term antibiotic treatment.
Decades ago, Prof Yue Wang and his team identified a cytosolic protein, Cyr1, the sole adenylyl cyclase, that acts as the cytosolic sensor of PGNs C. albicans. But a lingering mystery remained: How do PGNs get into the fungal cell to activate Cyr1?
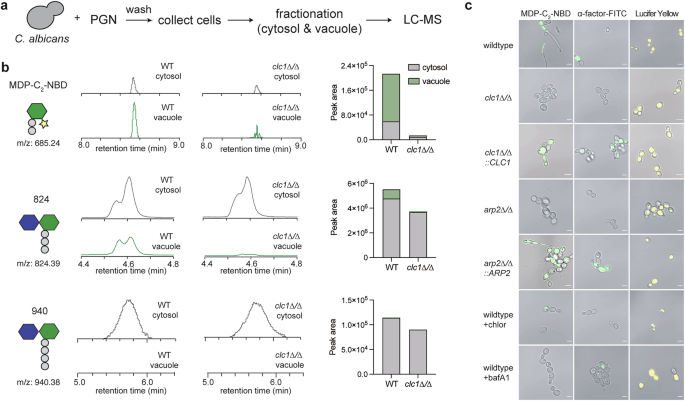
Our investigation began with our earlier curious observations that fluorescent PGN probes (e.g., MDP-C2-NBD) accumulate in the fungal vacuole (ACS Chem. Biol. 2022, 17, 9, 2538–2550). This led to our initial hypothesis that PGNs may be endocytosed into C. albicans, possibly facilitated by a cell surface PGN-specific receptor. Despite various screening and the application of chemoproteomic strategies, our efforts did not yield a receptor. We had always been cautious about the possibility that installing a fluorophore onto natural PGNs (of similar molecular weights) might potentially introduce artifacts. Consequently, we adopted an alternative approach to examine PGN uptake in C. albicans, employing LC-MS to monitor unlabeled, native PGNs.
Surprisingly, natural PGNs are localized primarily to the cytosol, not the vacuole, suggesting active or facilitated transport rather than endocytosis. The vacuolar accumulation of fluorescent PGN-probes was likely due to a regulatory process called ligand-induced endocytosis, used by yeast cells to modulate surface protein levels in response to excess ligands.
To identify the putative transporter responsible for cytosolic PGN uptake, we revisited a C. albicans strain (7KO) lacking all seven major peptide transporters (PTR2, PTR22, OPT1–5). Despite robust hyphal growth, the 7KO strain completely failed to import PGNs. This contradicted the assumption that PGN entry is required for morphogenesis through interaction with Cyr1.
A turning point of this project!
By reintroducing each transporter into the 7KO background, we identified Opt4 as the major PGN transporter in C. albicans. But what surprised us even more was the discovery that PGNs could still induce hyphal growth without entering the cell. This shifted our focus to the possibility of a membrane-localized PGN sensor in C. albicans.
We turned our attention to the Ssy1-Ptr3-Ssy5 (SPS) sensor complex, which is known to sense amino acids and activate the downstream signaling pathway. Interestingly, we found that expression of several OPT genes (OPT1-3) increased in response to PGNs in an Ssy1-dependent manner, hinting at direct PGN sensing by Ssy1. More inspiringly, we observed that the ssy1Δ/Δ mutant failed to form hyphae in response to various PGNs, despite still importing a moderate amount of PGNs into the cell, demonstrating that PGN uptake and PGN-induced morphogenesis are two independent processes.
To assess the physiological relevance of Ssy1 in vivo, we employed a β-lactam-induced PGN storm model in mice. Under the influence of PGNs, while wild-type C. albicans formed hyphae and disseminated systemically, the ssy1Δ/Δ mutant remained in the yeast form and was confined to the gut lumen. This strongly supports the idea that Ssy1 acts as a surface PGN sensor and presents a promising therapeutic target to curb antibiotic-associated Candida infections.
As a final reflection, beyond the scientific discoveries, this journey taught us something more: when experiments stall and data seem to contradict expectations, it’s useful to pause, re-evaluate, and be open to unexpected explanations. Some of the most exciting findings came not from charging ahead, but from questioning assumptions and embracing detours.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in