Asymmetric Diels-Alder reactions: A one-pot, multi-substrate screening approach
Published in Chemistry

Traditionally, organic chemists on the quest for new asymmetric, catalytic transformations rely on a so-called model substrate approach (a) – a long-standing strategy that led to numerous chemical reactions which have become indispensable to those engaged in synthesis. Unfortunately, scopes are occasionally rather narrow, i.e. only compounds resembling the model substrate may be transformed, which poses a severe constraint on the aforementioned strategy. In light of the shortcomings of this classical approach, Kagan et al. introduced the concept of one-pot, multi-substrate screening in 1998. [1] This contrasting strategy features the simultaneous exposure of a set of structurally distinct substrates to a catalyst in a single reaction vessel (b).
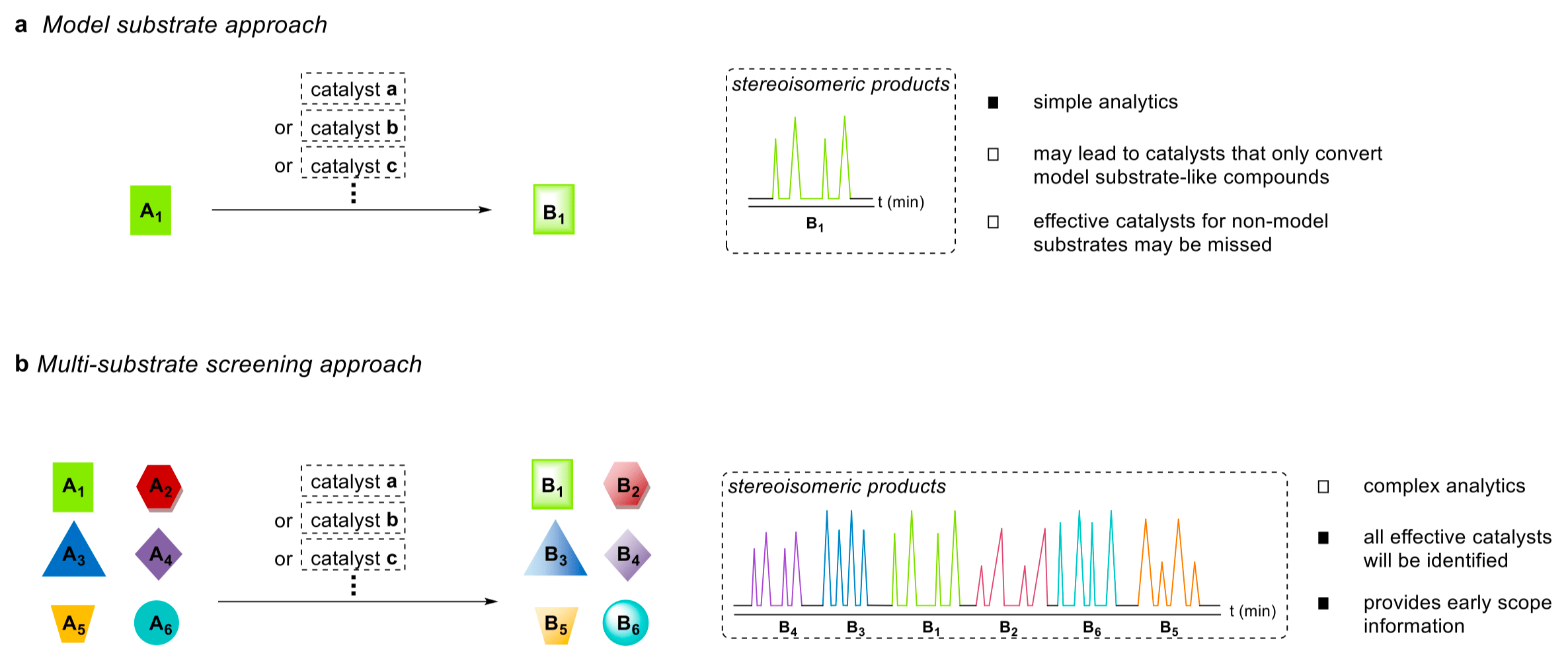
Fascinated by this alternative approach, we were determined to apply it in the laboratory, and had one of the most powerful transformations in organic chemistry in mind: the Diels-Alder reaction, or more specifically, the asymmetric [4+2] cycloaddition of cyclopentadiene and structurally diverse enals. The majority of established chiral catalysts for this reaction have one significant drawback, a stark bias for a particular substitution pattern of the aldehydic reaction partner. To identify a catalyst capable of accommodating the full range of enal substitution patterns, i.e. α-, β-, and α,β-disubstitution, we carefully chose a set of six representative enals that differed in the position, nature and size of their substituents.

The first obstacle we had to tackle was the establishment of an unambiguous analytical assay with the ability to baseline-separate all substrates and their corresponding stereoisomeric products. This complex analytical task, which can be quite tricky, is one of the major challenges of the one-pot, multi-substrate screening approach. We successfully surmounted this challenge as is demonstrated in the upper GC trace (racemic) below. With all the tools needed in hand, we began screening a multitude of different chiral Brønsted acid catalysts.

In this screening, the highly acidic and confined 3-phenanthryl substituted imidodiphosphorimidate (IDPi) showed superb reactivity and stereoselectivity (see the lower GC trace above). To further demonstrate the scalability and the robustness of the developed methodology, we conducted a multi-gram synthesis to afford >20 g of cycloadduct. Impressively, the catalyst was effortlessly recovered in near quantitative amounts by column chromatography without any loss in activity. It was ready for re-use after re-acidification with aqueous hydrochloric acid — thereby living up to its catalytic nature.
The present work has shown the one-pot, multi-substrate screening approach to be a valuable tool in methodology development. In our case, it facilitated the process of catalyst evaluation and ultimately led to the discovery and validation of a broadly applicable and highly stereoselective Brønsted acid catalyzed Diels-Alder reaction.
See for yourself, and read the full story here: Kim, H. et al. A multi-substrate screening approach for the identification of a broadly applicable Diels–Alder catalyst. Nat. Commun. 10, 770 (2019).
1. Gao, X. & Kagan, H. B. One-pot multi-substrate screening in asymmetric catalysis. Chirality 10, 120–124 (1998)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in