Lung structural cells are altered by influenza virus leading to rapid immune protection following re-challenge
Published in Biomedical Research, Immunology, and Anatomy & Physiology
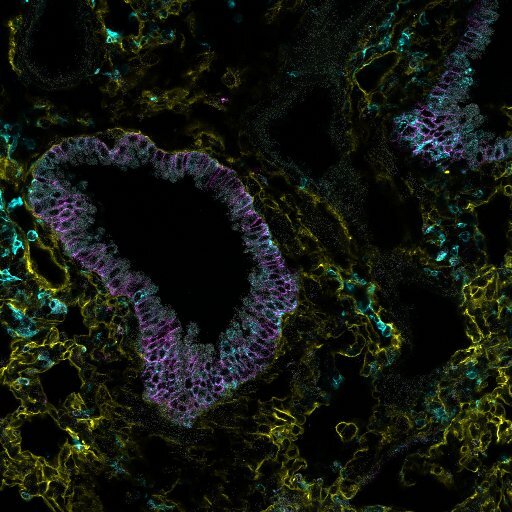
Classically, when we consider how the body remembers and then responds to infections, we think of the immune cells tasked with “fighting off” invading respiratory viruses. However, our recent study challenges this idea, revealing that the structural cells of the lung (epithelial cells, fibroblasts, and blood endothelial cells) not only survive this conflict but emerge in an altered state that contributes directly to lung immunity.
Do structural cells display immune memory?
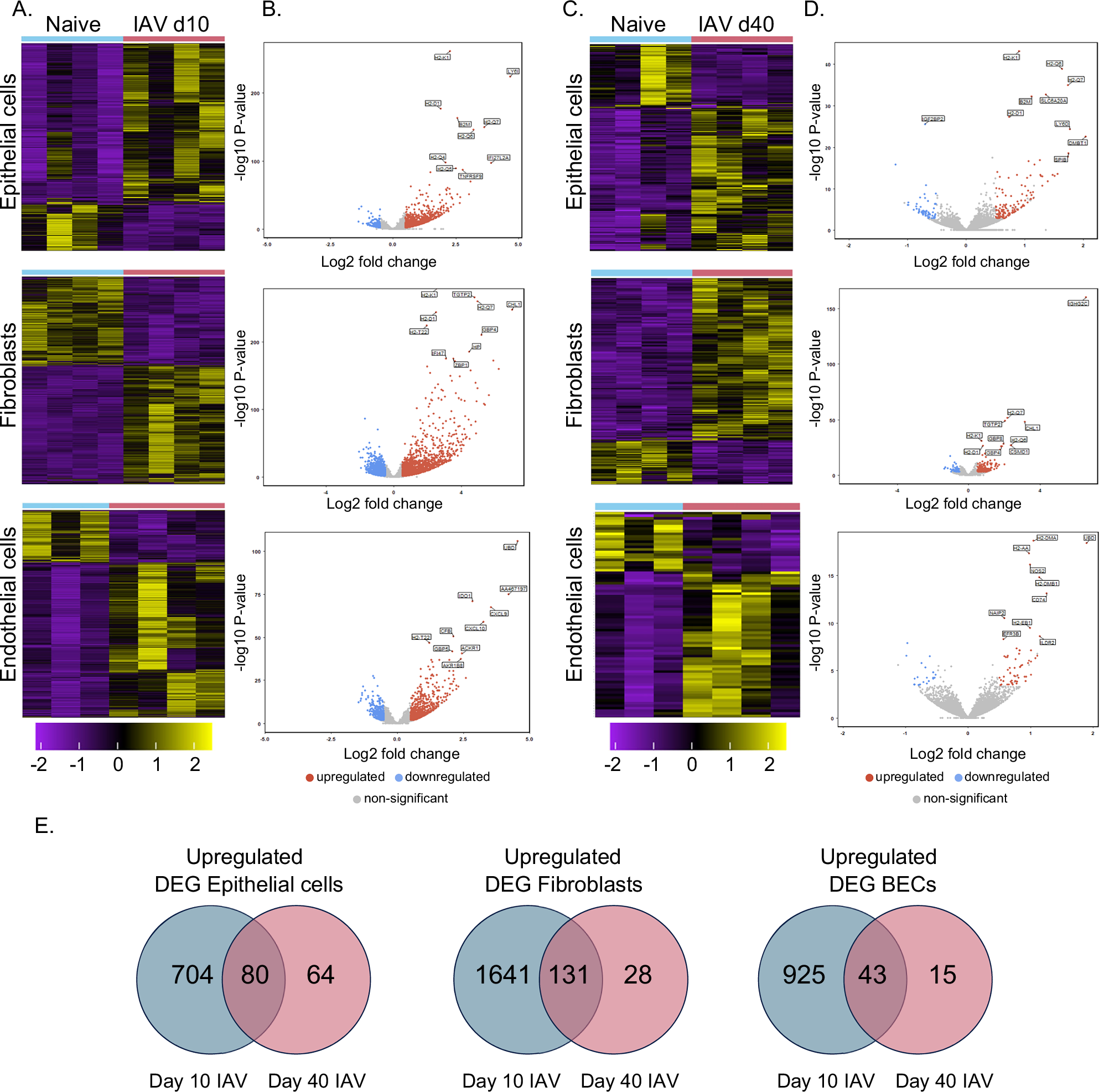
Our study used RNA-sequencing to demonstrate that following influenza A virus (IAV) infection, all three major structural cell types maintained sustained gene expression changes long after the virus was cleared. These changes included upregulated pathways for antigen processing and presentation, classically associated with ‘professional’ immune cells. Strikingly, these changes persisted for weeks positioning these lung cells to respond more effectively to a subsequent viral challenge.
Structural cells as active defenders of the lung
Historically, it has been considered that T cells play the dominant role in protection against different IAV strains. Our data challenge this idea, we show that lung structural cells, particularly epithelial cells, can restrict viral replication during re-infection with IAV. We used an in vitro assay (structural cell: T cell co-culture) to confirm that epithelial cells from previously infected mice controlled IAV more efficiently than those from naïve animals. This revealed enhanced anti-viral capacity and was accompanied by increased cytokine production, suggesting cell-intrinsic protective memory.
Fibroblasts and blood endothelial cells also showed lasting changes after infection, including engagement with T cells. This may be indicative of a coordinated, tissue-wide memory across the lung.
Is SpiB a putative transcriptional regulator of lung memory?
One of our most exciting findings was the role of the ETS transcription factor SpiB. This is classically associated with B, M, and plasmacytoid dendritic cells. SpiB was as an upstream regulator of the altered gene expression we observed in lung epithelial cells. In the tissue, we found that SpiB-positive epithelial were frequently located near persistent immune cell clusters. By generating a SpiB reporter mouse, we determined that SpiB+ cells expressed higher levels of MHC class I/II molecules. These SpiB+ cells, particularly in the upper airway, persisted for weeks post-infection, and may survive to rapidly alert the immune system to new threats.
Framing this in the context of epithelial cell memory
Our findings are consistent with previous observations that epithelial cells can adopt “trained/poised” states after injury or infection. Ciliated epithelial cells that survive IAV infection have previously been shown to persist with higher levels of MHCII, enabling more effective communication with T cells. Similar findings have also been reported in skin and gut epithelia, thus reinforcing the concept that structural cells can integrate past inflammatory experiences into subsequent responses.
Co-oridnation of the immune response: T cell (in)dependence?
At early time points after re-infection (day 2), we found that viral control was independent of T cells, implicating structural cells as major defenders during the initial viral response. Could these same cells enhance T cell activation and accelerate viral clearance later? Further investigation will be required to determine when these cells can act autonomously and in collaboration with immune cells.
Potential implications for vaccines and therapies
The discovery that structural cells harbour protective memory opens exciting avenues for vaccine development. If we could harness this from of tissue memory using adjuvants that activate fibroblast and epithelial responses we may be able to provide broader and longer-lasting protection against respiratory viruses. This does however raise the question of how widespread this phenomenon may be across tissues? And if infection with other respiratory viruses evokes a similar response (RSV, SARS-CoV-2)?
Future perspectives
Our findings suggest that lung structural cells function as much more than a passive scaffold, instead playing an active role in anti-viral defence, and are capable of “remembering” past infections at the cellular level. By uncovering key molecules (SpiB) that may co-ordinate this response, this brings us closer to designing interventions with the potential to leverage tissue memory for enhanced protective immunity.
The expanding field of structural immunology, while previously neglected, may help us to identify new allies in the fight against respiratory pathogens. We are only beginning to explore these possibilities.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
🔍 Ask the Editor – Clinical medicine, Respiratory physiology, and Cardiology
I’m excited to connect with a global network of specialists through the Research Communities – how will you get involved?
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in