Biological evolution is constrained by geochemistry: a molybdenum case study
Published in Ecology & Evolution and Microbiology
Imagine a beautiful summer day at the beach with your feet on sand submerged under blue waters. The sky is blue and palm trees and dolphins jumping are visible in the distance. This is perhaps more reminiscent of a marketing pitch for a beverage company, time share operation, or tourism push plays out today. Now, flip the calendar back around 2.5 billion years. It’s a hot summer day on a newly emerged continent. You’re standing on thick granite, with little sand to be seen at what would someday become a beautiful beach. You try to take a deep breath, but you begin to choke due to the lack of oxygen in the orange red-tinted skies above you. As you look around all you see is a barren land mass and an ocean that is green and without visible life, just odd rocks standing in the tide.
Of course, this latter scene is less than ideal for achieving rest and relaxation. Let’s be honest, most people are more excited by a jumping dolphin than seeing stromatolites slowly growing over decades in the tides. But for the microorganisms that call that stromatolite home, it is clear that things are about to change. The proliferation of oxygenic photosynthesis and the oxygen that they produce would soon change the planet and that home for those stromatolites. It is hard to exaggerate the impact that the production of oxygen on our globe truly had, as today it is near inescapable with the dominance of aerobic lifeforms that surround us, including animals, trees, and most of the “model” microorganisms that are studied. With so much contradictory evidence of what life would have been life 2.5 billion years ago , it is hard to imagine how it might have operated and thrived.
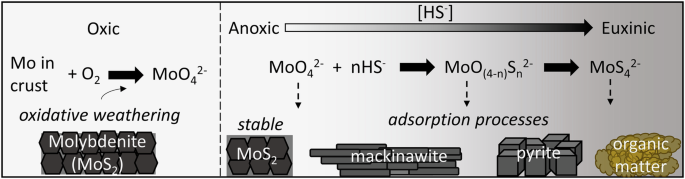
The release of O2 initiated a series of changes on Earth. In the case of three elements that are required by most organisms - iron, sulfur and molybdenum- the change was vast. We went from green iron-rich oceans to characteristic blue oceans after precipitating the iron out as rust (seen as banded iron formations). Similar to iron being oxidized, so too were other redox sensitive elements like sulfur and molybdenum. Pyrite (iron disulfide) and molybdenite (molybdenum disulfide) exposed on continental surfaces began to be weathered by oxygen, delivering sulfate and molybdate to the oceans in vast quantities like never before. Thus, unlike Fe which became less bioavailable, elements like S and Mo became more available as oxyanions. In the case of Mo, this change resulted in molybdate (MoO42-) becoming the most abundant trace element in modern oceans.
Molybdate availability and usage has been extensively studied due to it being an essential cofactor in enzymes that govern the carbon, sulfur, and nitrogen biogeochemical cycles. Perhaps most interesting is its integration in the biological enzymatic machinery (called nitrogenase) used to produce ammonia (a nitrogen-based fertilizer) by “fixing” nitrogen gas in the atmosphere. This process, carried about by microscopic bacteria and archaea, is responsible for the fertile soils that all forms of higher life now depend on. This has led plants and nitrogen-fixing bacteria to have evolved a mutually beneficial relationship wherein plants (such as legumes) feed newly fixed carbon to the bacteria that are localized in “nodules” in the roots in exchange for ammonia.
One important paradigm that has formed about the use of molybdenum is that molybdate is the only bioavailable form of this element. This is a “fact” you see repeated in textbooks, scientific articles, and farming blogs. However, this is primarily from studies of aerobic organisms, those cells that rely on oxygen to power their metabolism and are the cornerstone of “life as we know it.” A clue that this may not be the whole story came from nitrogenase and its evolutionary history… it evolved over 2 billion years ago (based on numerous phylogenetic studies) in an anaerobic methanogen.
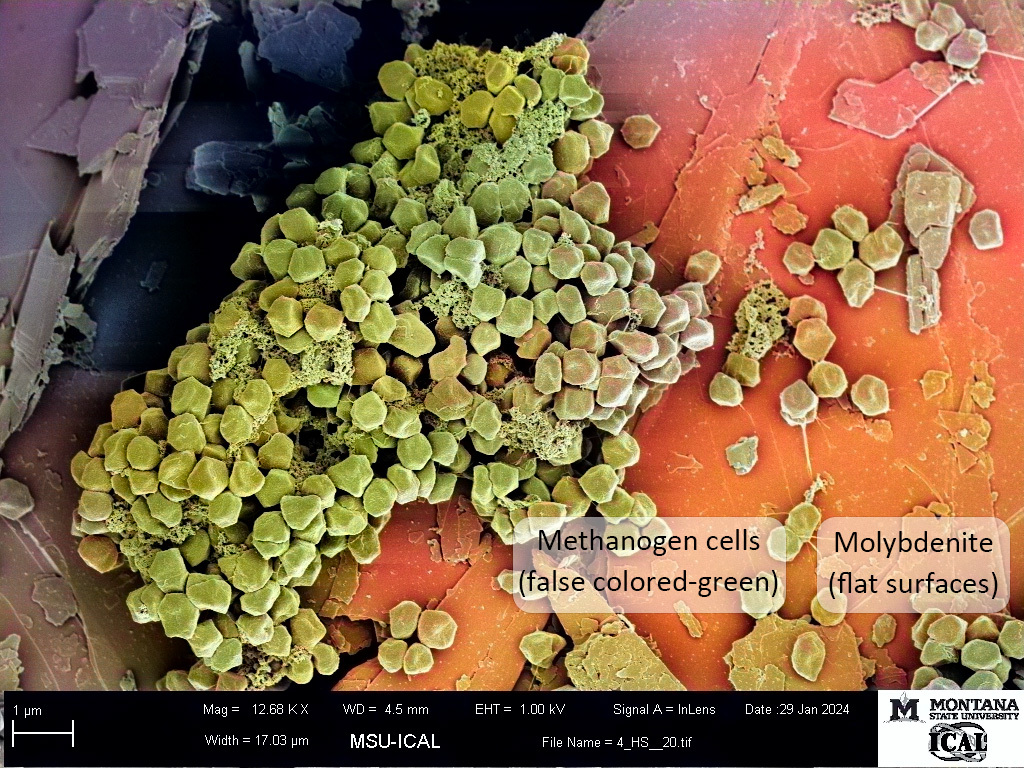
Methanogens are single-celled archaea that have existed for even longer periods of time, upwards of 4 billion years depending on the record that one is studying, long before oxygenic photosynthesis evolved. They are found in subsurface environments rich in sulfide and sulfide minerals--far from the direct reach of oxygen. Our group has been focusing on how these cells acquire iron and sulfur from pyrite, and what benefits this may provide the cell. In an earlier article published in Nature Communications Biology (Payne et al., 2023), we showed that cells grown on pyrite while fixing nitrogen required far less molybdate than cells grown with iron and soluble sulfide. We observed that when molybdate is mixed with sulfide, it quickly reacts to form tetrathiomolybdate (MoS42-). The conversion of molybdate to tetrathiomolybdate seemed like a plausible explanation for the poor growth with sulfide--it fit the paradigm of molybdate being the critical bioavailable form of molybdenum. But something bugged us about this result… how could the earliest cells with nitrogenase have evolved at a time when molybdate wasn’t yet available?
We decided to do some digging and found that molybdenite and tetrathiomolybdate could be sources of molybdenum found in hydrothermal environments inhabited by methanogens. At the time, we could find no papers that directly tested the use of tetrathiomolybdate or molybdenite as a molybdenum source under anaerobic conditions. Interestingly though, tetrathiomolybdate has been studied as a therapeutic for copper toxicity in ruminants.
This spurred further research, leading to experiments where we demonstrated that tetrathiomolybdate is very reactive with metal components in our growth medium. Perhaps this is why it was never truly tested as a molybdenum source? Thus, we went through a year of optimizing and modifying our growth medium to preserve either tetrathiomolybdate or molybdate to compare how cells grow on either source. In the process, we discovered that our model methanogen organism could actually use a combination of two sulfur sources never shown to support growth, a bonus discovery, which enabled our experiments to move forward.
With our approach, we were able to demonstrate that molybdate and tetrathiomolybdate support growth equally well for our methanogens. This also extended to cells provided with molybdenite. These data are extremely exciting for us as geobiologists that firmly believe that biology and geochemistry must be synchronized as they advance. In a way, it seems obvious - of course an anaerobe would be able to use key elements in the forms most relevant to their environment! Paradigm broken.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in