
I was firstly exposed to two-dimensional (2D) layered halide perovskites semiconductor materials in early 2013 when Dr. David Mitzi visited UCLA and delivered an excellent seminar (I was a PhD student with Prof. Yang Yang and later jointly with Prof. Fred Wudl). Originally trained as a polymer chemist, I thought inorganic materials are not easily tunable and the materials chemistry is far less rich than organic ones. However, I was deeply amazed by the highly tunable structures and the excellent electronic properties that the perovskite scaffold offers after Prof. Mitzi’s talk and after reading some literatures by several other pioneers from Europe and Japan.
The layered halide perovskite has the general chemical formula of L2An-1BnX3n+1, where L is a large cation (mostly large-size or long-chain organic cations, such like butyl ammonium C4H9NH3+ [BA+]), A is a monovalent cation such as Cs+ or MA+, B is a divalent metal cation such as Pb2+ or Sn2+, and X is a halide. The variable n which indicates the number of the metal halide octahedron layers between the two L cation layers. When n = 1, the structure constitutes an ideal quantum well with 1 atomic layer of [BX6]4- separated by the L cations.
My journey with 2D halide perovskites started in 2015 when I was a postdoctoral fellow at UC Berkeley with Prof. Peidong Yang. As I was starting a new position and trying to look for new directions, we noticed the growing interests in halide perovskite materials and 2D materials. We generated an idea to synthesize the first atomically thin (one unit cell thick) 2D halide perovskite crystals (BA)2PbBr4 using a fully solution processing (Science 2015). This was exciting. However, the poor environmental stability of the thin perovskite sheets limited their applications in electronic and optoelectronic devices. Then, my research shifted to and focused on inorganic nanowires at Berkeley.
I revisited this area when I started my independent career at Purdue University’s Davidson School of Chemical Engineering in 2017. The goal was to combine my knowledge and skill sets from both of the organic and the inorganic materials research to design new 2D hybrid materials with better properties. The first thing we wanted to do was to incorporate conjugated organic semiconductor building blocks into the 2D perovskite lattice (to replace the insulating butyl ammonium groups) to synthesize organic – inorganic hybrid quantum wells with widely tunable properties. In addition, the bulky and rigid conjugated molecules are expected to protect the moisture sensitive metal halide layer better and therefore improve the materials’ stability. It turned out that this was not an easy task at beginning, because the large conjugated organic molecules tend to aggregate and phase-separate from the hybrid lattice. In this Nature Chemistry paper, we describe an effective molecular design strategy to overcome this challenge and we demonstrate a variety of such hybrid quantum well structures with tunable optical properties and dramatically improved stability (Figure 1).
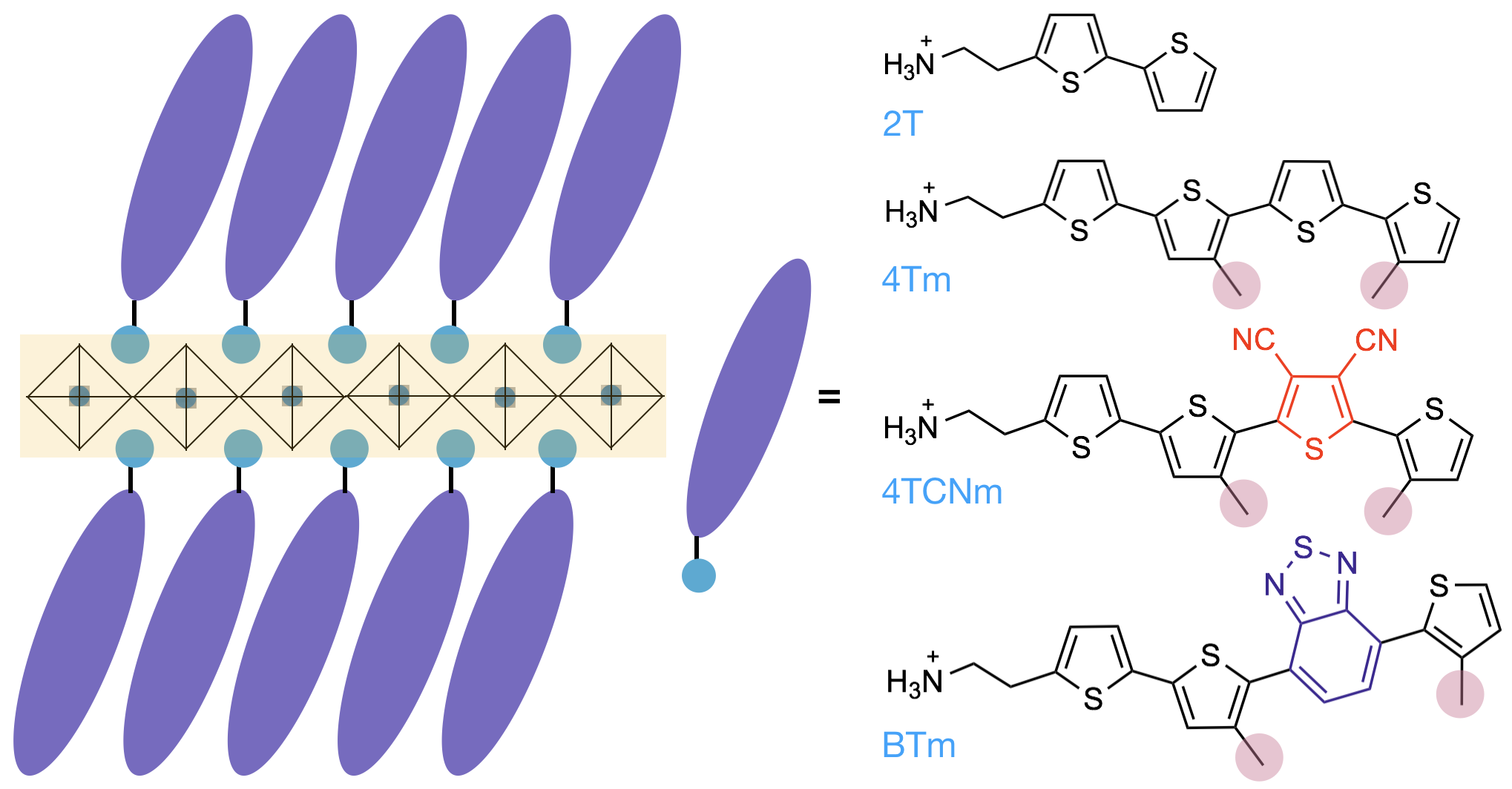
Figure 1. General structural illustration of hybrid perovskite quantum well (L)2PbI4, where L is an organic semiconductor building block. (Nature Chemistry 2019)
Now, this work immediately opens up a wide chemical space for designing functional organic building blocks for hybrid perovskites, including but not limited to semiconducting, conducting, light-emitting, optically active building blocks. The practical applications of such hybrid materials are also very broad. For example, a high-performance perovskite field-effect transistor has been demonstrated in our recent JACS paper. In addition, quantum well light emitting diodes, lasers, and photodetectors based on these types of materials are also expected to have superior performances. The chemistry and physics associated with the organic – inorganic interfaces are fundamentally interesting as well.
Read our paper in Nature Chemistry: https://www.nature.com/articles/s41557-019-0354-2
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in