Carbon dioxide conversion to hydrocarbons: thinking big to see small things
Published in Chemistry

The corresponding Nature Communications paper is here: http://go.nature.com/2Fra0zd
From a chemical viewpoint, recycling and efficiently converting waste carbon dioxide into useful products is one of the challenges in modern chemistry; carbon dioxide is very stable! But converting it could give power generation a carbon-neutral environmental footprint. We make use of a variety of imaging techniques, synchrotron based techniques and density functional theory (DFT) simulations to develop a molecular level understanding of Fe(III) oxyhydroxide on nitrogen-doped carbon materials active in the carbon dioxide electroreduction, learning what it should look like to work efficiently, and paving the way for future research that can make it work with higher productivity and long-term stability.
I came across this topic very early in my scientific career, as a student of the International Max Planck Research School (IMPRS) at the Fritz Haber Institute of the Max Planck Society. Within the ELCASS project and as part of a doctoral training programme of the European Research Institute of Catalysis (ERIC), we were investigating how to enable the carbon-carbon coupling reaction electrocatalytically starting from carbon dioxide as feedstock to produce long chain hydrocarbons. If we could realize this reaction with high efficiency, productivity and maximum chemical control, then it could be an alternative, sustainable route for small-scale chemicals synthesis.
The idea was to use iron-based materials because iron is a known active material in the synthesis of long chain hydrocarbons from carbon monoxide and molecular hydrogen (Fischer-Tropsch synthesis).
I was intrigued during my PhD to observe what I later called the nitrogen effect, that by doping the carbon support with nitrogen it was possible to obtain an iron-based electrode more active for the carbon-carbon coupling from carbon dioxide reduction than the iron on the nitrogen-free carbon. Despite the low overall productivity and poor efficiency obtained in the early days, deciphering what the nitrogen effect means in chemical terms was the quest.
This was basically a prelude to the work published today in Nature Communication, which spans many years and several institutions and countries.
Indeed, this paper is an example of a large scientific community collaborating and making extensive use of large scale facilities: Italy (University of Messina); The UK (Johnson Matthey Technology Centre in Reading, University of Reading, University of Southampton), Diamond Light Source, UK Catalysis Hub at the Research Complex at Harwell, UCL); Germany (FAU Erlangen-Nürnberg and the Max Planck Institute for Chemical Energy Conversion and BESSY II). In addition to large-scale experimental facilities, our work also used the UK national supercomputing facility ARCHER for quantum-mechanical simulations.
In this study, we explore the carbon dioxide reduction reaction activity of iron oxyhydroxide nano-structures (ferrihydrite-like structure) supported on oxygen- and nitrogen-doped graphite supports in a low concentration bicarbonate solution, and are able to optimize the experimental conditions and identify conditions of very high efficiency of the process.
By means of operando Q-EXAFS (Quick-scanning Extended X-ray absorption fine structure) carried out at the Core X-ray Absorption Spectroscopy (B18) beamline of Diamond Light Source it was possible to reveal the reversible red-ox chemistry of iron oxyhydroxide nanostructures on nitrogen-doped carbon in low concentration bicarbonate solution, characterized by the formation of Fe(II) species at cathodic potentials relevant for carbon dioxide reduction, whereas at more negative potentials those species turn into metallic iron. The unwanted molecular hydrogen evolution from water is indeed correlated to the transformation into metallic iron.
We prove that a chemical interaction is favoured between iron terminal sites of oxyhydroxide phase and the pyridine nitrogen species on the carbon surface. Joint research on Johnson Matthey’s aberration corrected transmission electron microscope within the electron Physical Science Image Center (ePSIC) at Diamond Light Source enabled us to visualize single atoms and clusters of iron localized at the edge of the layers of the graphite structure, where nitrogen functionalities exist. In the following images the tiny entities of iron, which account for the carbon dioxide reactivity are found in the scanning transmission electron micrograph (in the background) decorating the edge of the graphene layers of the graphite structure (white spots in the red square). The structural model on top of the images is meant to reproduce in 3D what is observed in the electron micrograph.
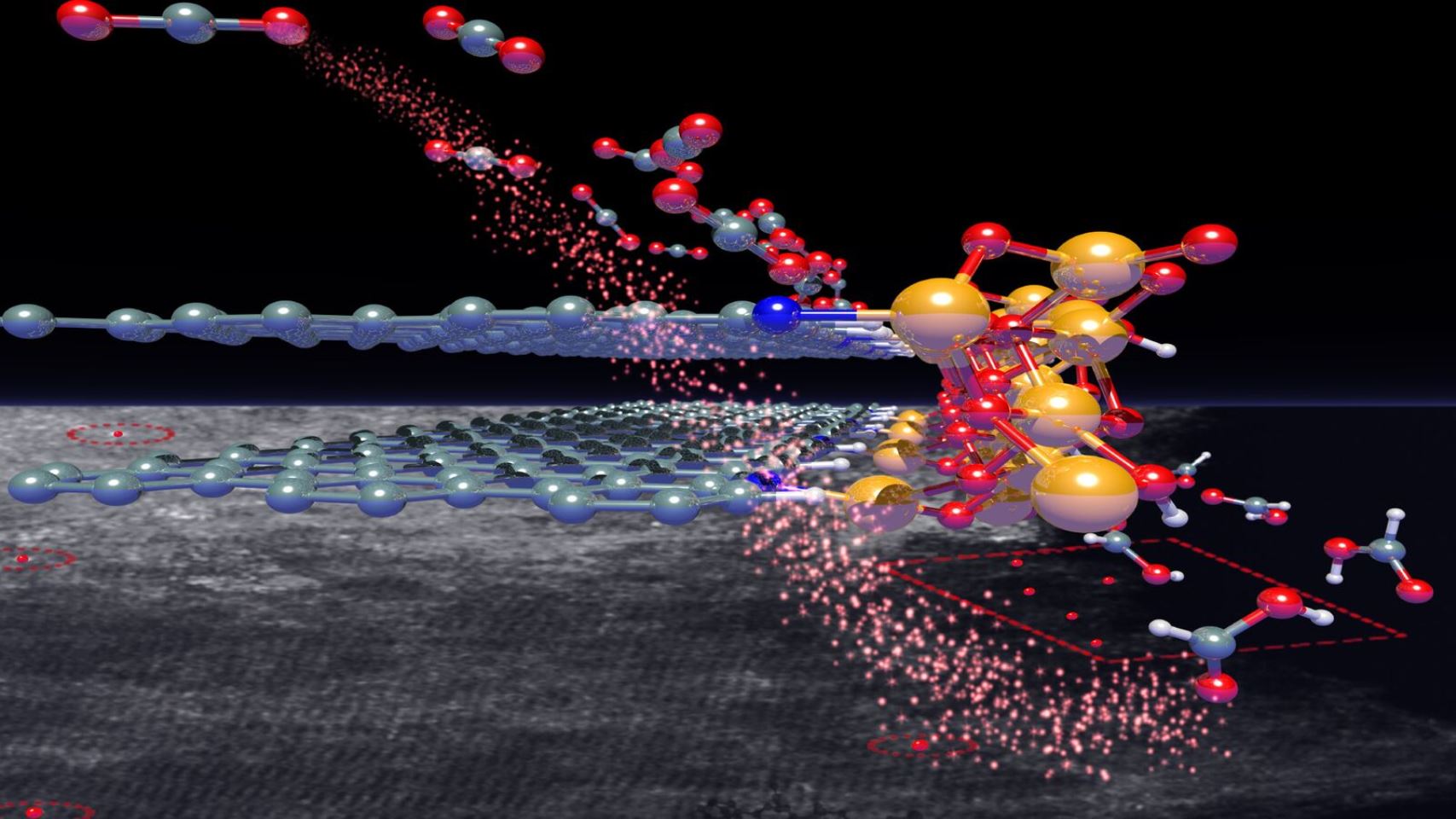
Image caption: scanning transmission electron micrograph (ePSIC facility) and model of the ferrihydrite decorating the graphite edges. Color code: Fe atoms (orange); C atoms (grey); Oxygen atoms (red), N atoms (blue). Image credit: Victor Posligua, Rosa Arrigo, Manfred Erwin Schuster.
It is due to the nitrogen species and the favorable iron–nitrogen interaction that Fe(II) species are initially present on the starting material in very low amount but also are further formed at carbon dioxide reduction reaction relevant potential. We found that both Fe(II) and nitrogen sites can interact with bicarbonates ions: this is the first step towards their reduction and coupling. It’s a small part of a big move towards being able to make the chemicals we need in a more environmentally-friendly way, recycling carbon dioxide and powering the chemical reactions with renewable energy.
We found some surprising parallels between the way that ferrihydrite works in the catalyst and its role in our planet’s iron cycle, the pathways through which iron moves in carbonated environment (in changing forms through a dissolution and precipitation mechanism) through the biosphere and Earth’s various environments. Chemical elements are used and reused over and over again, and this supply of iron is essential for all living things. So while our work focuses on the minute details of chemical reactions, we know it’s always part of a much larger picture. It is quite comforting that by observing nature we might find inspiration.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in