Chemical catalyst manipulating cancer epigenome and transcription —Toward a new era of “catalysis medicine”—
Published in Cancer and Cell & Molecular Biology
In life, countless chemical reactions are facilitated by enzymes, which act as natural catalysts. The functions of fundamental components of life—primarily biomacromolecules such as proteins, nucleic acids, and lipids—are defined by their chemical structures. Biological activities generally arise from the interactions and chemical reactions among these molecules. However, when specific chemical reactions become excessive or deficient, they can lead to disease. Many small-molecule inhibitors currently used as drugs exert their therapeutic effects by binding to enzymes and inhibiting their functions, thereby suppressing the abnormal chemical reactions underlying disease. Despite significant advances in drug development, including small-molecule drugs and biopharmaceuticals, effective treatments for certain conditions, such as cancer and neurodegenerative diseases, remain elusive.
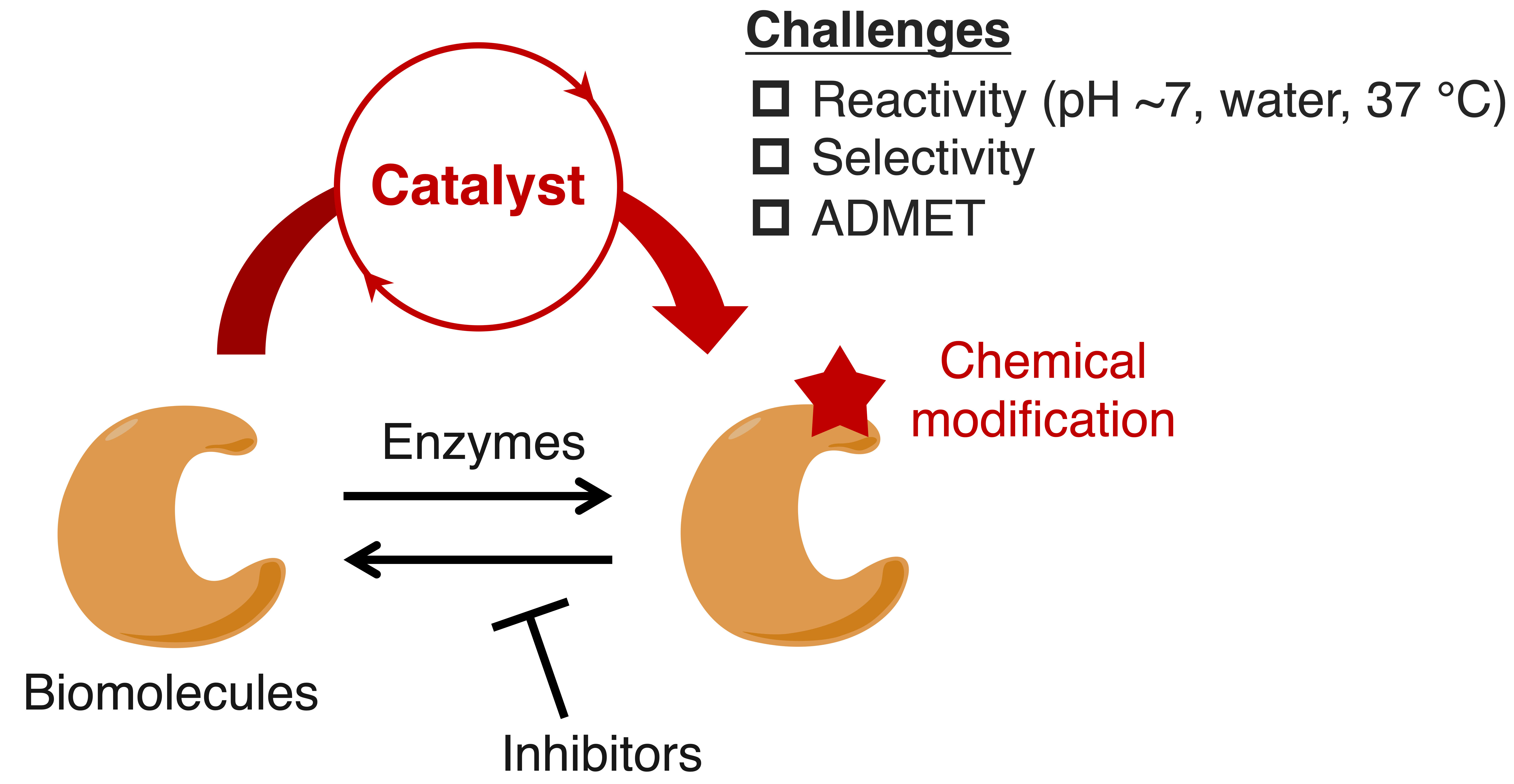
Over the past decade, our group has introduced the concept of “catalysis medicine,” wherein chemically synthesized catalysts promote (i.e., catalyze) intracellular chemical reactions to complement or even surpass the functions of endogenous enzymes (Figure 1). These chemical catalysts have the potential to represent a new class of medicines by enabling specific chemical reactions that traditional small-molecule inhibitors cannot achieve. Ultimately, such catalysts could not only treat diseases but also “evolve” normal biological processes into a healthier state. However, realizing this vision requires addressing several challenges. Catalysts must operate under mild physiological conditions —neutral pH, aqueous environments, and body temperature (37 °C)— while selectively modifying target molecules and avoiding nonspecific reactions with abundant off-targets, such as water, biological thiols like glutathione (GSH), and other biomolecules. Additionally, pharmacological considerations, including absorption, distribution, metabolism, excretion, and toxicity (ADMET), are critical for in vivo applications. Progress in this field will also drive innovation in organic synthesis, promoting the development of “bio-friendly” chemistry with reduced environmental impact.
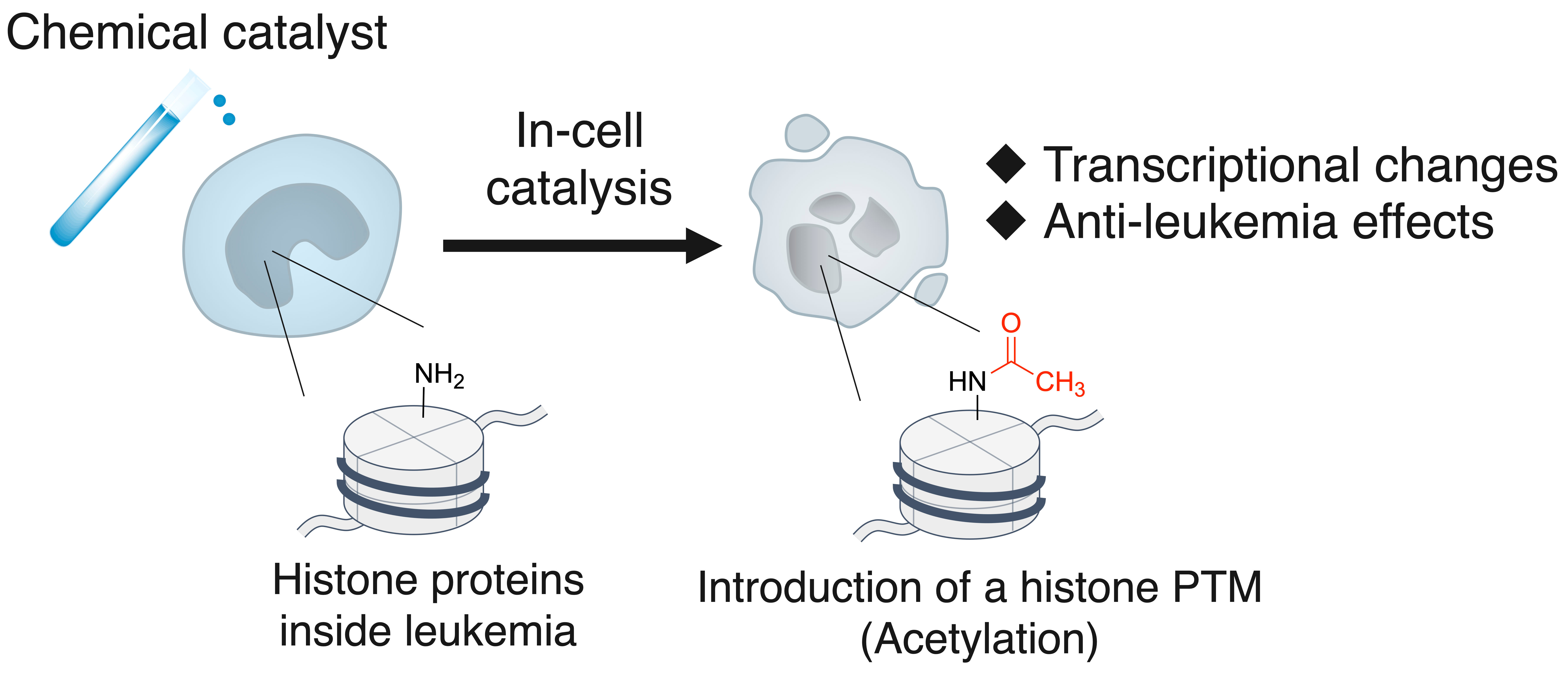
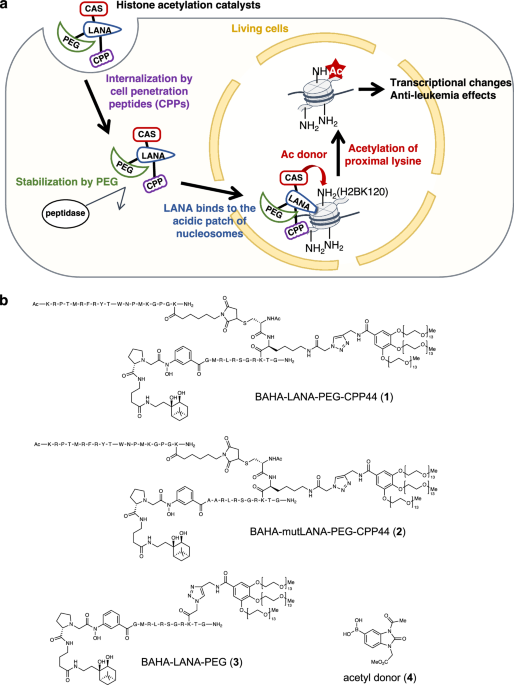
In this study, we developed a chemical catalyst capable of modulating the epigenetic landscape in cancer cells, leading to transcriptional changes and anti-leukemia effects (Figure 2). In eukaryotic cells, histone proteins package DNA into nucleosomes, and these histones, along with DNA, undergo various post-translational modifications (PTMs), such as acetylation and methylation, collectively forming the epigenome. The epigenome plays a crucial role in dynamically regulating chromatin structure and transcription. Aberrations in the epigenome are often implicated in diseases such as cancer, making the targeting of cancer-specific epigenomes a prominent anti-cancer strategy. For instance, histone deacetylase (HDAC) inhibitors are clinically used as anti-cancer drugs. However, these inhibitors face several challenges, including off-target effects due to the broad specificity of histone-modifying enzymes, the presence of undruggable targets, and the emergence of drug resistance. In contrast, our approach employs a histone-modifying chemical catalyst that functions independently of endogenous enzymes. This unique mechanism circumvents these limitations, providing a promising therapeutic strategy against cancer.
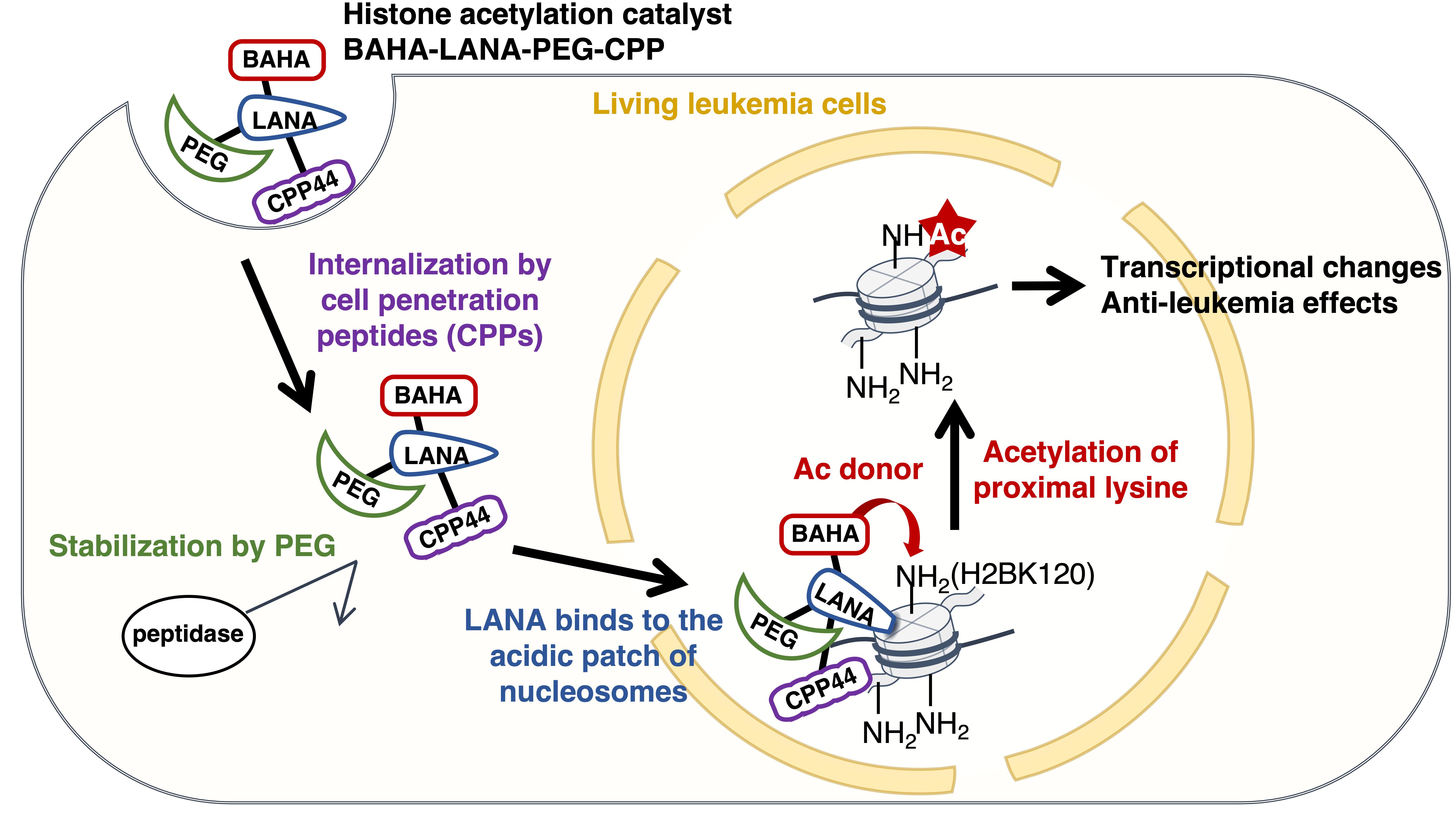
In 2021, our group reported a histone acetylation catalyst capable of functioning in living cells. However, its low cell-permeability and limited acetylation activity posed significant challenges for further application. To address these issues, we developed a novel catalyst, BAHA-LANA-PEG-CPP (Figure 3). This catalyst selectively enters leukemia cells via CPP44, a leukemia-specific cell-penetrating peptide. The polyethylene glycol (PEG) moiety shields the LANA peptide from peptidases, thereby enhancing its stability within cells. The LANA peptide binds to a specific region of nucleosomes known as the acidic patch, while the BAHA (boronate-assisted hydroxamic acid) moiety—an advanced catalytically active site we previously developed—facilitates the acetylation of the proximal H2BK120 residue within a short reaction time (~60 minutes). Furthermore, we observed that H2BK120 acetylation mediated by our catalyst reduced leukemia cell viability and tumorigenic potential in mice. Transcriptome and epigenome analyses revealed that H2BK120 acetylation weakened the chromatin binding of the negative elongation factor E (NELFE), an oncogenic transcription factor.
This study represents the first demonstration of therapeutic effects achieved by directly intervening in in-cell epigenomes using an entirely chemical approach with an artificial catalyst. It marks a significant milestone in advancing the concept of “catalysis medicine”—a pioneering strategy that employs chemical catalysts to regulate biological reactions and treat diseases. Building on this foundation, we are expanding this approach to target other types of post-translational modifications across various proteins, further advancing the field of catalysis medicine toward broader and more transformative applications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in