Corepressor protein activity regulation by SliM-induced polymerization
Published in Cancer and Cell & Molecular Biology

This contribution is a long-standing collaboration between two research organisations; European Molecular Biology Laboratory (EMBL) and University Medical Center Hamburg-Eppendorf (UKE) Hamburg combining structural biology with tumor biology to investigate a new mechanism of regulation in cancer progression. The mechanism involves well-known corepressor scaffold proteins (CtBP1 and CtBP2) and a functionally unknown intrinsically disordered protein (IDP) (RAI2). Since one third of the human genome represent IDPs, one would wonder about their impact on cellular processes. This is precisely what we decipher in our contribution showing CtBP activity regulation through RAI2-induced polymerization. The CtBPs bind through a single canonical PxDLS-like motifs on their target proteins, but our interest piqued upon identification of tandem non-canonical identical motifs (ALDLS) on RAI2. Understanding the relevance of this observation required stable recombinant RAI2 protein, which would then lead to biophysical and structural characterization.
RAI2 is a 530 amino acid protein that lacks secondary and tertiary structure over the complete length of its sequence. Not surprisingly, the initial attempts to recombinantly produce RAI2 protein were unsuccessful and hence, we decided to focus on obtaining tandem motifs containing truncated RAI2 proteins. This in general was also an uphill task considering their susceptibility to degradation. After almost two years of trying different construct lengths, tags and expression hosts, the first breakthrough was achieved upon shifting the focus to the recombinant overexpression of a 60-amino acids stretch of RAI2 in E.coli as host. This peptide along with the two less stable 163 and 227 amino acid proteins enabled us to perform a biophysical characterization of their complex with CtBPs, in addition to obtaining a crystal structure with one of the ALDLS motifs. During this investigation, we observed for the first time that RAI2 induces well-ordered polymerization in CtBP using negative stain electron microscopy. On the tumor biology front, the CtBP-RAI2 interaction leads to the formation of nuclear foci in breast cancer and prostate cancer (PC) cells. These foci disappear when one or both ALDLS motifs have been mutated.
Over the period of next two and half years, our innumerable attempts to reproduce these well-ordered polymers for obtaining high-resolution cryogenic-EM structure remained unsuccessful. This was attributed to the presence of inherent disorder in the truncated RAI2 proteins. At this point, when we were ready to give up on obtaining high resolution EM structure, the second breakthrough was achieved when I truncated the 21 amino acid linker length between the two ALDLS motifs by removing 10 amino acids from the linker. This paved the way for high resolution cryo-EM structure determination of RAI2-induced CtBP polymerization that is more rigid than the wild type complex.
CtBPs are corepressor scaffold proteins that recruit transcription factors and chromatin modifiers leading to gene repression. Their overexpression has been associated with several forms of cancers and are known to antagonize several tumor suppressor genes. The observation that RAI2-induced CtBP polymerization lifts the repression on a known CtBP target leads to a hypothesis that RAI2 disassembles the CtBP corepressor complex on the target gene (Figure 1). The hypothesis is further fueled by the colocalization of other repression machinery components with CtBP/RAI2 nuclear foci. Further research is required to better understand the mechanism and circumstances that lead to RAI2-induced CtBP polymerization.
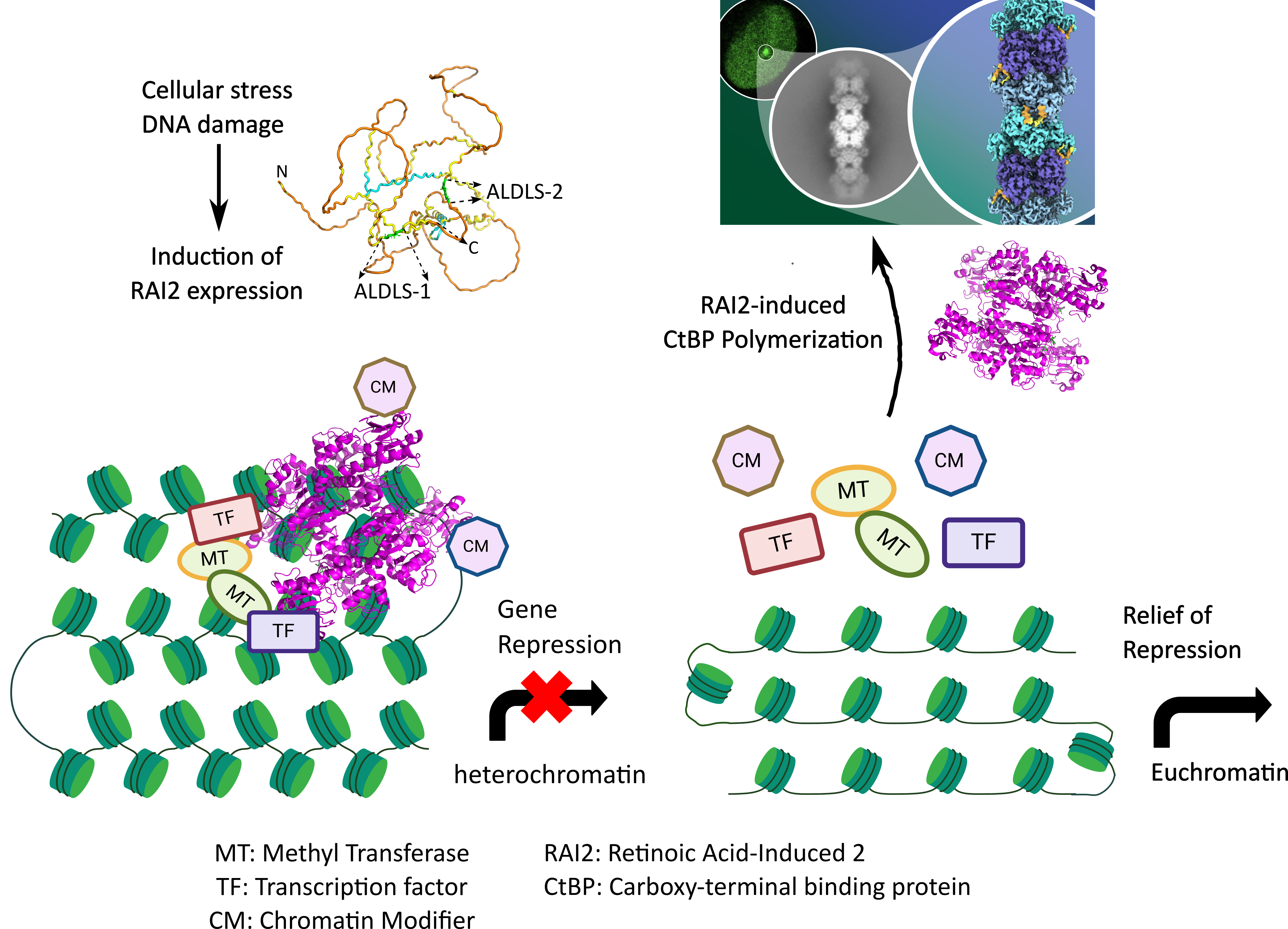
Figure 1: Tetrameric CtBPs contribute to multiprotein complexes that include transcription factors, methyl transferases and chromatin modifiers, leading to the formation of heterochromatin and ultimately gene repression. In response to cellular stress and DNA damage, expression of RAI2 is induced. RAI2 through its tandem ALDLS motifs binds CtBP, induces polymerization and snares CtBPs from the corepressor complex. This disassembly of corepressor complex is associated with the relief of repression of specific genes implicated in cancer progression, most likely through a transition of heterochromatin to euchromatin. Elements of the figure were prepared with Biorender.
The analysis of the CtBP/RAI2 complex in a parental and RAI2 KO PC cell line revealed markers for neuroendocrine features, which are found in aggressive forms of PC. This prompted our investigation into RAI2 expression in circulating tumor cells (CTCs) from PC patient blood samples. The presence of CTCs in the blood are indication of metastasis. The PC cell line observations were mirrored in the blood samples wherein the aggressive forms of PC were associated with diminishing levels of RAI2.
Overall, the current observations suggest that RAI2 might function as a tumor suppressor protein through its direct interaction with CtBPs, but this requires further scrutiny. Lastly, the inactivation of the CtBP corepressor activity by leveraging the presence of tandem PXDLS-like motifs sets up for an exciting future in the field of translational cancer research.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in