Crosstalk between porcine circovirus and the host cells
Published in Microbiology, Cell & Molecular Biology, and Zoology & Veterinary Science
The complex relationship between viruses and their hosts drives the evolution of defense mechanisms in both. While the mechanisms of infection and replication are similar among the majority of studied viruses, there is substantial evidence of variation in these processes due to interactions with the hosts. Therefore, further studying these interactions is crucial for developing antiviral strategies and addressing new viral threats.
Porcine Circovirus 2 (PCV2) is a tiny virus that causes significant economic damage to the global swine industry. It has a compact DNA genome and mainly targets the swine immune system. Due to its small genome, PCV2 replication is considered to be heavily dependents on the immune cell machinery for replication, and can hide within them, such as macrophages and dendritic cells, altering their function1. However, the details of how PCV2 maintains a persistent low-level in target cells are poorly understood.
Our study published in Communications Biology, titled "Retinol binding protein 4 restricts PCV2 replication via selective autophagy degradation of viral ORF1 protein", reveals a key mechanism in PCV2 infection. We found that retinol binding protein 4 (RBP4), an adipokine for retinol carrier, is rapidly produced through activation of the MAPK-eIF4E signaling pathway in host cells in response to PCV2 infection. The increase in RBP4 triggers the autophagic degradation of a key PCV2 protein, ORF1, limiting the virus replication. This suggests that RBP4 could serve as a host defensive measure against PCV2, while the virus also hijack this process to maintain a dynamic balance with the host immune system.
Autophagy is critical in immune defense and viral infection. Viruses can block autophagy to replicate, while the host can use it to degrade viral proteins2. Our research show that RBP4 activates the E3 ubiquitin ligase TRAF6 and SQSTM1/p62, which promotes K63-linked ubiquitination of the viral replication-associated protein ORF1 for degradation. To the best of our knowledge, this is the first report that selective autophagy plays a critical role in the modulation of PCV2 infection. We found that RBP4 triggered SQSTM1/p62-mediated selective autophagy through the activation of TLR4 signaling. Given that TRAF6 is the downstream kinase of the TLR4 signaling, it is not surprising that RBP4 promotes activation of the E3 ligase TRAF6. Therefore, our results identified a previously unappreciated mechanism for TRAF6 in the regulation of selective autophagy and viral protein ubiquitination.
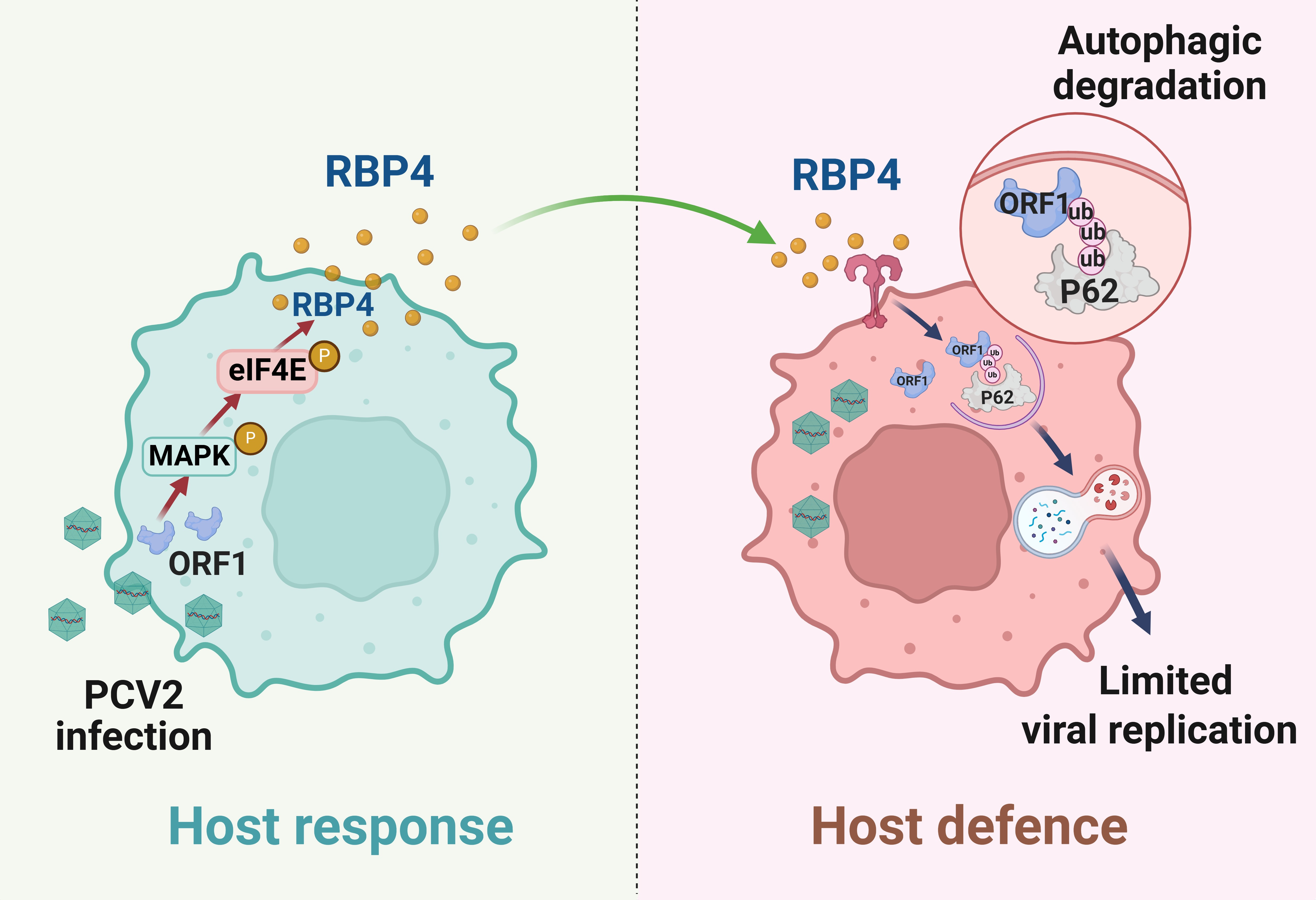
Figure 1. A schematic model of the interaction between PCV2 and host cells.
Circoviruses, including PCVs, have circular, single-stranded DNA and can infect various hosts. While there is no definitive evidence of PCVs infecting humans, their presence has been detected in human samples3. Recently, a new human circovirus, HuCV2, closely related to PCV3, has been identified, sparking concerns regarding its origins, prevalence, and potential pathogenicity in humans4. As such, controlling PCV infections is crucial for the well-being of both humans and animals. A significant challenge in PCV2 replication is the low viral titer in cells, which limits vaccine production. This reduced reproductive rate appears to be a common trait among some circoviruses, likely due to their intricate interactions with host cells. Our study reveals that RBP4 plays a pivotal role in suppressing PCV2 replication by targeting the degradation of ORF1. Given the structural similarities between PCV2, PCV3, and HuCV2, it is plausible that key host factors may regulate the productive replication of all circoviruses. Our findings not only illustrate how a host restriction factor can modulate circovirus replication but also highlight a critical mechanism for the interaction between circoviruses and their host cells.
In conclusion, our study has shown that the host factor RBP4 can activate the selective autophagy pathway to control PCV2 replication. This discovery provides a foundation for developing strategies to combat circoviruses and potentially create vaccines.
References
- X.J. Meng. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system, Annual review of animal biosciences. 1, 43-64 (2013).
- Y. Choi, J.W. Bowman, J.U. Jung. Autophagy during viral infection - a double-edged sword, Nat. Rev. Microbiol. 16, 341-54 (2018).
- J. Denner, A. Mankertz. Porcine Circoviruses and Xenotransplantation, Viruses. 9, (2017).
- Y. Li, P. Zhang, M. Ye, R.R. Tian, N. Li, L. Cao, Y. Ma, F.L. Liu, Y.T. Zheng, C. Zhang. Novel Circovirus in Blood from Intravenous Drug Users, Yunnan, China, Emerg. Infect. Dis. 29, 1015-9 (2023).
Preparation of the figures was aided by BioRender.com.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in