Cryo-EM structure of the CDK2-cyclin A-CDC25A complex
Published in Cell & Molecular Biology

Introduction
The cyclin-dependent protein kinases (CDKs) are essential regulators of the eukaryotic cell cycle, requiring association with their cognate cyclin partners and post-translational modifications for activity[1,2]. Notably, phosphorylation of a conserved tyrosine (Tyr15 in human CDK1) within the glycine-rich loop (G-loop) of the ATP binding site, inhibits CDK1/2 activity.
CDC25 (M-phase inducer phosphatase) was first identified in Schizosaccharomyces pombe to promote entry into mitosis by removing inhibitory CDK1 Tyr15 phosphorylation [3,4]. In humans, three CDC25 isoforms A, B and C[5-7] cooperate to regulate CDK activity[8] by removing Tyr15 phosphorylation on CDK1/2. Specifically, CDC25B activates CDK1-cyclin B complexes at G2/M[9], whilst CDC25A dephosphorylates CDK2-cyclin A/E complexes during G1/S[10-12]. The CDC25 phosphatases also play a key role in response to DNA damage[13,14].
Changes in CDC25 expression are frequently observed in a variety of cancers, often in correlation with poor prognosis[15]. This has led to considerable interest in small-molecule orthosteric inhibitors and allosteric compounds to target CDC25-protein interactions[16,17]. However, the development of these approaches has been limited by the absence of any structure of a CDC25 isoform in complex with a CDK-cyclin module.
Here, we determined the structure of the ~ 86 kDa CDK2-cyclin A-CDC25A complex by cryogenic electron microscopy (cryo-EM) to 2.7 Å resolution (PDB 8ROZ, EMD-19408).
Cryo-EM Structure Determination
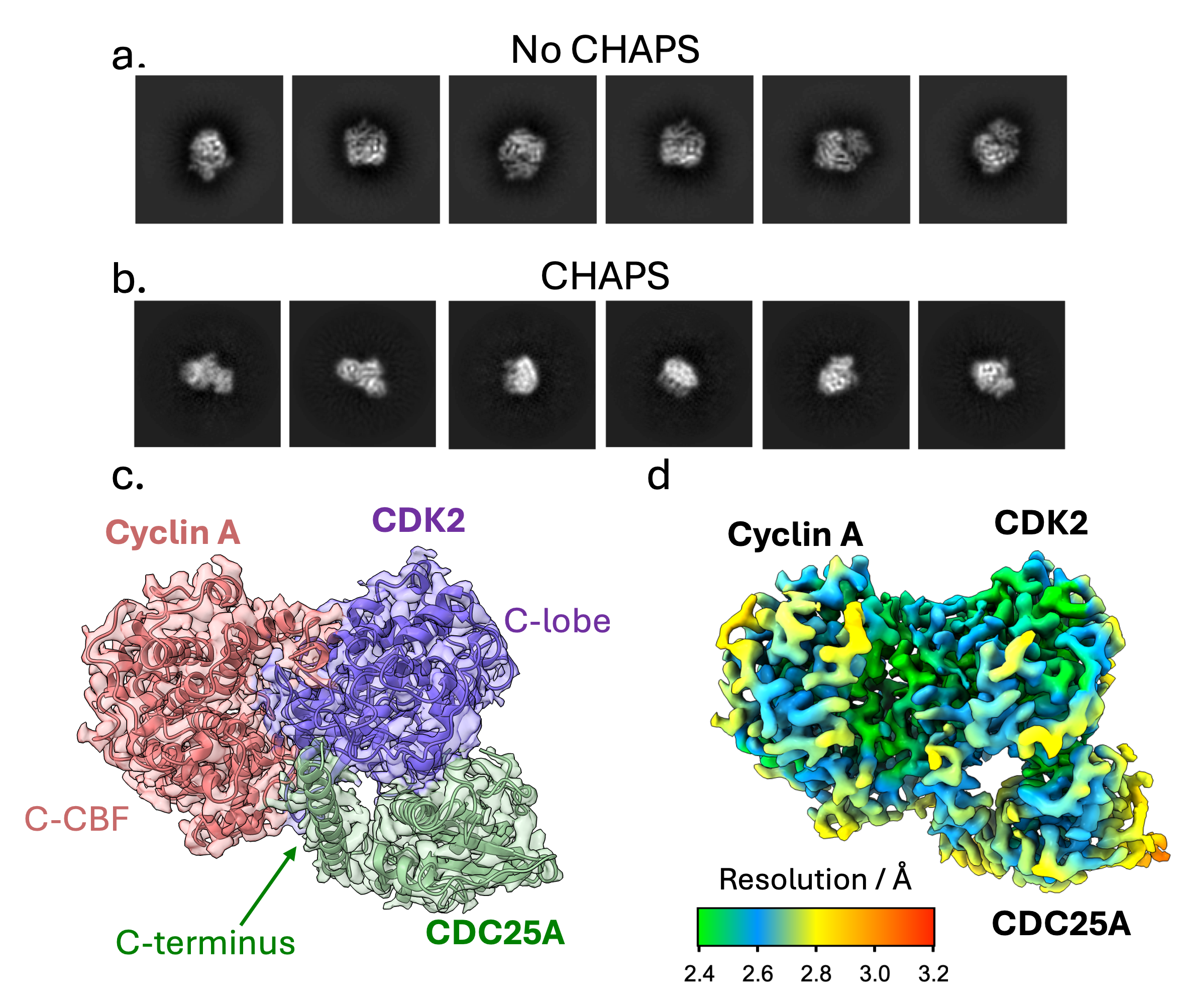
To generate a stable ternary complex for cryo-EM studies, CDK2 was phosphorylated on Tyr15 and Thr160, and CDC25A was catalytically inactivated by C431S mutation. During preliminary data collection, we encountered difficulties with preferential orientation, Figure 1 a, resulting in poor 3D refinement. A suitable specimen was subsequently obtained by spiking the complex with CHAPS detergent to improve the orientation distribution, Figure 1 b. High-resolution data collection was performed on a 300 kV FEI Titan Krios and data were processed to yield a 2.7 Å map, showing clear density for the quaternary architecture, secondary structure, and side chain organisation of the ternary complex, Figure 1c-d.
Architecture of the CDK2-cyclin A-CDC25A Complex
Within the ternary complex, CDK2 has its characteristic β-sheet N-terminal domain and predominantly helical C-terminal lobe, which bind the two helical domains, N- and C-terminal cyclin box folds (N- and C-CBF), of cyclin A to form an extensive protein-protein interface
The CDC25A catalytic domain comprises a central 5-stranded parallel β-sheet enclosed by 5 α-helices, which bridges the bi-lobal structure of CDK2. The mutated catalytic residue (C431S) initiates the conserved protein tyrosine phosphatase (PTP) loop, forming a shallow active site that recognises phosphorylated Tyr15 (pTyr15) of CDK2. The structure further reveals a previously unrecognised CDC25A C-terminal helix (residues 495-524) which binds at the CDK2-cyclin A interface, providing a mutual binding site in the trimeric complex.
CDC25A-CDK2 interactions: CDC25A substrate recognition
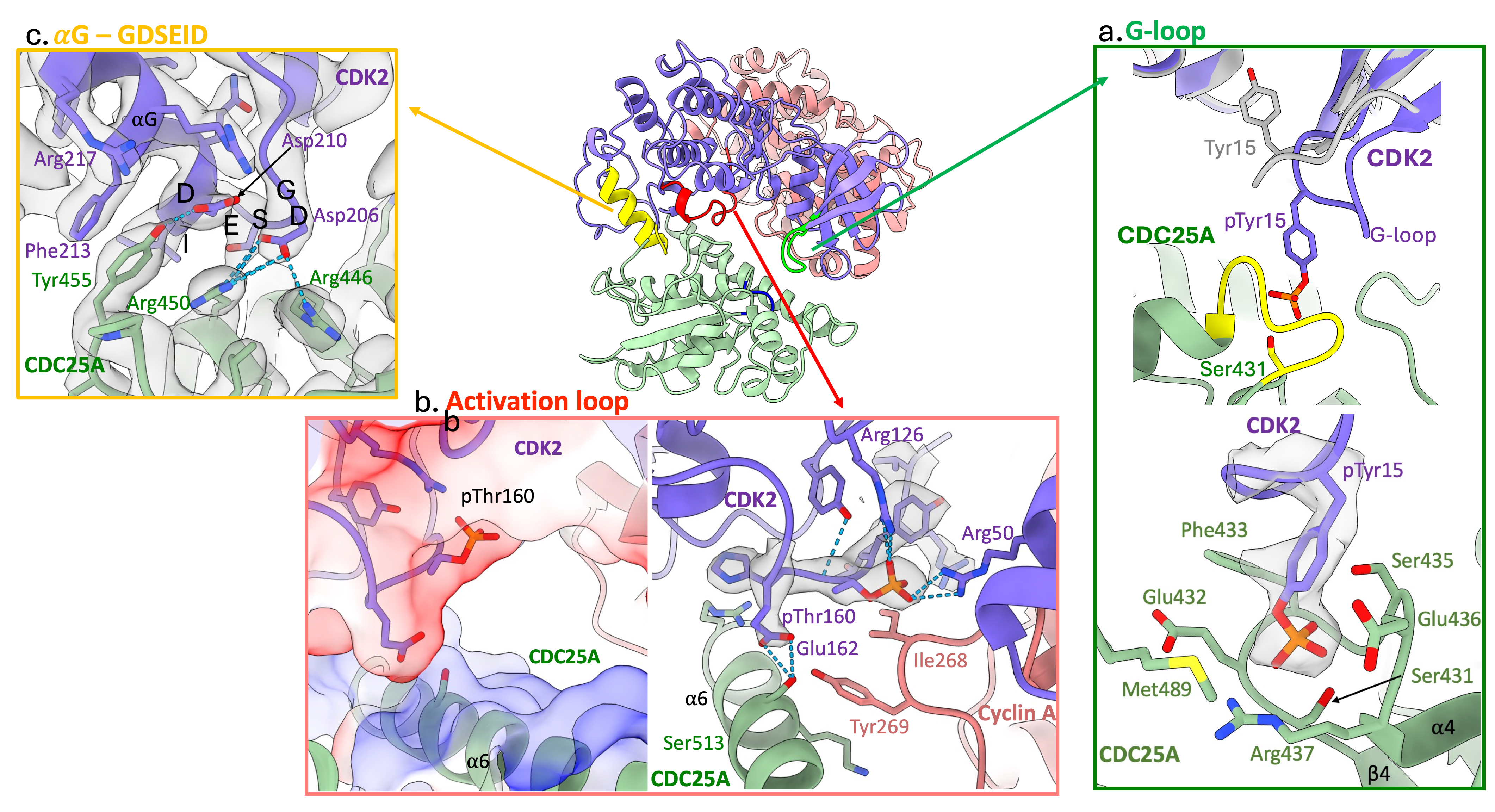
Our cryo-EM structure reveals that substrate recognition by CDC25A is driven by the CDK subunit through three distinct CDK2 regions;
- G-loop: in the N-terminal lobe, contains pTyr15 (depicted in green in Figure 2)
When bound to CDC25A, the G-loop is drawn away from the surface of CDK2 to position pTyr15 into the active site of CDC25A, Figure 2 a.
- Activation segment: in the C-terminal lobe, contains pThr160 (depicted in red in Figure 2)
The activation segment provides a negatively charged surface that binds the opposingly charged CDC25A C-terminal helix, which in turn, engages with the C-CBF of cyclin A, Figure 2 b.
- GDSEID-αG helix motif: in the C-terminal lobe (depicted in yellow in Figure 2)
The GDSEID (single letter amino acid code) sequence and processing αG helix form various hydrogen bonds, salt bridges and π-stacking interactions to secure the binding of the CDC25A catalytic core with the C-terminal lobe of CDK2, Figure 2 c.
We further verified these regions of CDK2-CDC25A interaction through hydrogen-deuterium exchange mass spectrometry.
CDC25A-cyclin A interactions
CDC25A makes relatively few contacts with cyclin A, interacting solely with the C-CBF through its C-terminal helix. Using surface plasmon resonance we demonstrate that the affinity of CDC25A(C431S) for pT160CDK2-cyclin A (Kd ~ 15 µM) is significantly greater than for monomeric CDK2 (pT160 and pY15pT160) for which Kd values could not be reliably determined. Consequently, despite its limited binding profile, cyclin A has a significant role in facilitating trimeric complex formation by enforcing conformational changes in CDK2 to make it a CDC25A substrate.
Importance of the CDC25A C-terminal helix
The CDC25A C-terminal helix is not visible in the monomeric CDC25A crystal structure (PDB 1C25[18]), indicating this region may be disordered, transitioning to nascent secondary structure on binding CDK2-cyclin A. Additionally, this C-terminal region is known to mediate interaction with 14-3-3 proteins[19], indicating it is flexible in solution to promote binding to the 14-3-3 recognition cleft. This further supports our hypothesis that the C-terminal region of CDC25A is structurally dynamic.
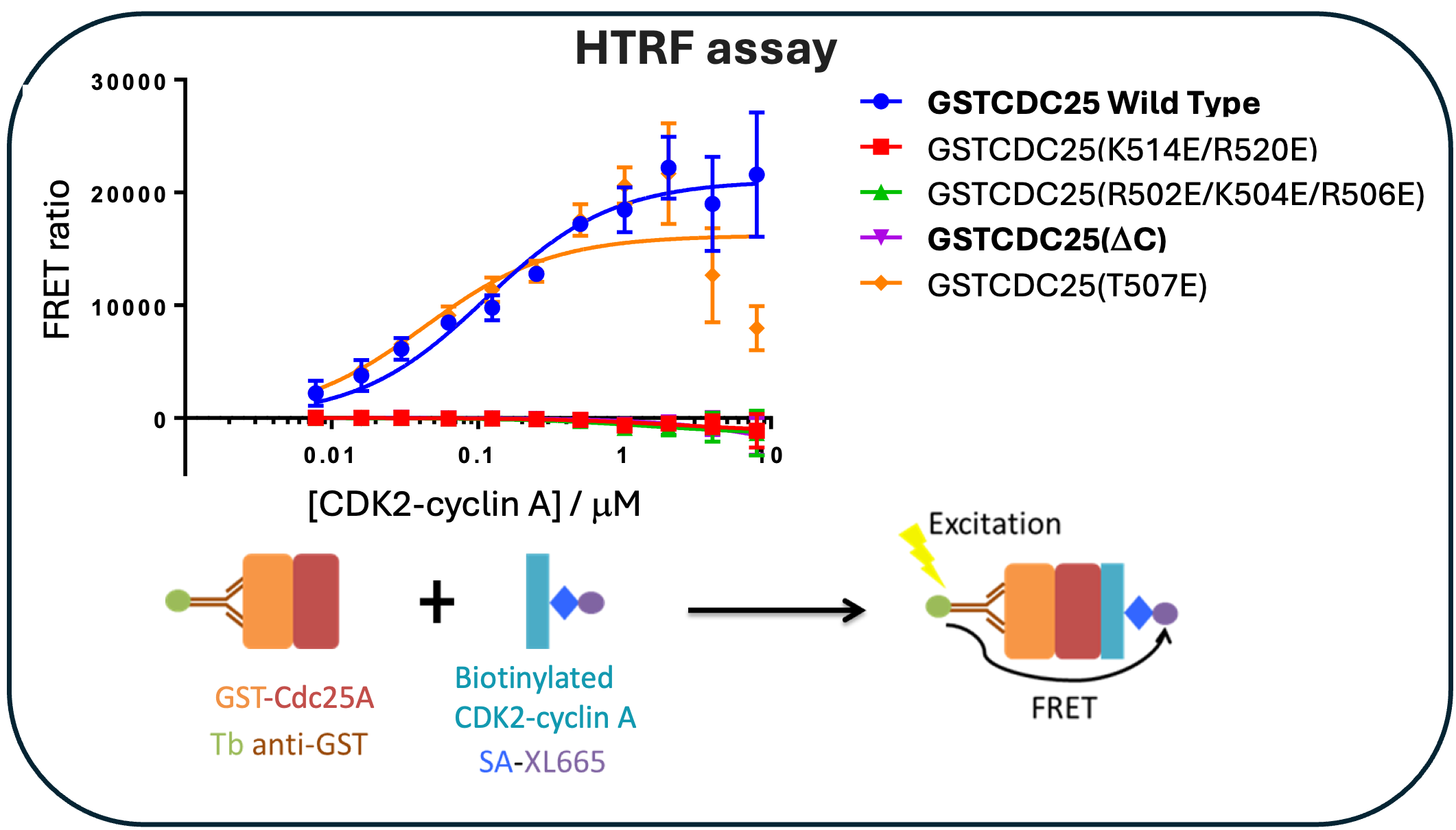
Through homogenous time-resolved fluorescence (HTRF), we demonstrate that removal of the C-terminal tail or charge reversal mutations in the C-terminal sequence abrogates binding with CDK2-cyclin A, Figure 3, confirming the requirement for the CDC25A C-terminal region in complex formation.
Importance of the CDK2 GDSEID sequence
There are now structures for three protein complexes that exploit the CDK2 C-terminal lobe for complex formation; CDK2 bound to cyclin-dependent kinase subunit 1 (CKS1, PDB 1BUH [20]), kinase-associated phosphatase (KAP, PDB 1FQ1 [21]) and CDC25A. Structural comparisons reveal the CDK2 GDSEID motif is an important binding site for these protein partners, conferring selectivity by engaging different residues and hydrophobicity surface profiles. The fact the GDSEID sequence is a hotspot for protein binding provides a mechanistic explanation for the identification of yeast cell cycle mutants within the sequence[22].
Potential to investigate CDC25 substrate preferences
Using our cryo-EM structure, we identified key regions that mediate complex formation and performed sequence conservation analysis across the CDK, cyclin and CDC25 families to identify potential partner preferences. This analysis suggests CDK1/2-cyclin A, CDK1-cyclin B and CDK2/3-cyclin E complexes are suitable partners for CDC25A, supporting its role in G1/S and G2/M transitions. However, CDK4-cyclin D complexes appear unlikely substrates. We further probed this using AlphaFold Multimer[23], which accurately predicted the experimental structure of CDK2-cyclin A bound to CDC25A, but struggled to confidently model binding to CDK4-cyclin D3. This work provides a structural basis for further experiments to establish the importance of CDK4 inhibitory phosphorylation and the role of CDC25 phosphatases.
Future Considerations
As key cell cycle checkpoint regulators, altered CDC25 expression is frequently identified in human cancers with poor prognosis. Consequently, the CDC25 isoforms are attractive anticancer drug targets. Previous docking simulations and fragment-based studies have provided proof-of-concept in targeting the CDC25-CDK interactions. We envisage this cryo-EM structure will facilitate the identification of allosteric sites amenable to the development of inhibitors to prevent complex assembly or disrupt CDC25A catalysis.
References
- Morgan, D. O. CYCLIN-DEPENDENT KINASES: Engines, Clocks, and Microprocessors. Annu Rev Cell Dev Biol 13, 261–291 (1997).
- Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9, 153–66 (2009).
- Russell, P. & Nurse, P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145–53 (1986).
- Gould, K. L. & Nurse, P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342, 39–45 (1989).
- Gautier, J., Solomon, M. J., Booher, R. N., Bazan, J. F. & Kirschner, M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67, 197–211 (1991).
- Dunphy, W. G. & Kumagai, A. The cdc25 protein contains an intrinsic phosphatase activity. Cell 67, 189–96 (1991).
- Nagata, A., Igarashi, M., Jinno, S., Suto, K. & Okayama, H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol 3, 959–68 (1991).
- Boutros, R., Dozier, C. & Ducommun, B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 18, 185–91 (2006).
- Lindqvist, A., Källström, H., Lundgren, A., Barsoum, E. & Rosenthal, C. K. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol 171, 35–45 (2005).
- Hoffmann, I., Draetta, G. & Karsenti, E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J 13, 4302–4310 (1994).
- Jinno, S. et al. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J 13, 1549–56 (1994).
- Blomberg, I. & Hoffmann, I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol 19, 6183–94 (1999).
- Sanchez, Y. et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–501 (1997).
- Karlsson-Rosenthal, C. & Millar, J. B. A. Cdc25: mechanisms of checkpoint inhibition and recovery. Trends Cell Biol 16, 285–92 (2006).
- Sur, S. & Agrawal, D. K. Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol Cell Biochem 416, 33–46 (2016).
- Abdelwahab, A. B., El-Sawy, E. R., Hanna, A. G., Bagrel, D. & Kirsch, G. A Comprehensive Overview of the Developments of Cdc25 Phosphatase Inhibitors. Molecules 27, (2022).
- Rudolph, J. Inhibiting transient protein-protein interactions: lessons from the Cdc25 protein tyrosine phosphatases. Nat Rev Cancer 7, 202–11 (2007).
- Fauman, E. B. et al. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell 93, 617–25 (1998).
- Chen, M.-S., Ryan, C. E. & Piwnica-Worms, H. Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding. Mol Cell Biol 23, 7488–97 (2003).
- Bourne, Y. et al. Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 84, 863–74 (1996).
- Song, H. et al. Phosphoprotein-protein interactions revealed by the crystal structure of kinase-associated phosphatase in complex with phosphoCDK2. Mol Cell 7, 615–26 (2001).
- Ayscough, K., Hayles, J., MacNeill, S. A. & Nurse, P. Cold-sensitive mutants of p34cdc2 that suppress a mitotic catastrophe phenotype in fission yeast. Mol Gen Genet 232, 344–50 (1992).
- Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat Methods 19, 679–682 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in