Deciphering Bat Influenza: New Insights into H18N11 Infection Dynamics
Published in Microbiology

Introduction
In a previous study, we showed that the bat influenza A virus (IAV) H18N11 uses major histocompatibility complex II (MHC-II) for cell entry. This was surprising because all conventional IAVs use sialic acid for cell entry. This shift to using a proteinaceous receptor instead of glycans intrigued us. Classic IAVs mainly infect epithelial cells, but these cells rarely express MHC-II. So, which cell types does the virus infect?
Working with Tony Schountz in Fort Collins, who maintains a colony of Jamaican fruit bats thought to be a natural host for H18N11, we showed that infection occurs mainly in the gut and is associated to with immune-associated tissues such as Peyer's patches and tonsils. We observed infected epithelial cells, as well as infected cells located in the lamina propria. However, due to the lack of specific antibodies, we were unable to determine the exact cell types at that time. In this new study, we again collaborated with Tony Schountz and investigated the infection dynamics of H18N11 in Jamaican fruit bats at the single-cell level. We provide a detailed view of how this virus interacts with the bat immune system, identify the infected cell types, and discuss the possible implications for a better understanding of zoonotic potential.
Study Design
We infected Jamaican fruit bats with two variants of the bat IAV H18N11: the wild-type virus (WT) and a mutant lacking the non-structural protein 1 (ΔNS1). NS1 of IAV is known to play a crucial role in antagonizing the host immune response, thereby facilitating viral replication. We then processed the intestine and mesentery of these bats at 3 and 9 days post-infection for single-cell RNA sequencing (scRNA-seq), including mock-infected controls. With the help of Geoffroy Andrieux, a highly skilled bioinformatician from Freiburg, we were able to decipher the large single-cell sequencing dataset.
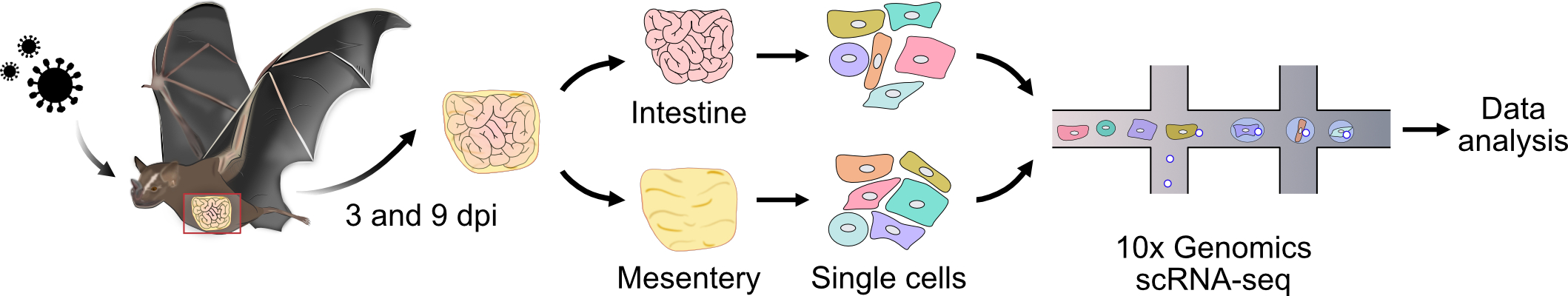
Key Findings
-
Cellular Landscape of Bat Intestine and Mesentery: The bat intestine and mesentery are populated primarily by canonical immune cell types and some epithelial cells. Notably, there is a cluster of cells with both T cell and NK cell characteristics (NK/T cells). Similar cell populations have been described in Egyptian rousette bats and Cave nectar bats, suggesting NK/T cells may be a general component of the bat immune repertoire.
-
Infection Dynamics: Jamaican fruit bats exhibit an overall relatively mild antiviral transcriptional response to H18N11 infection, which is most pronounced in macrophages and B cells. There is also a notable increase in the expression of activation markers in neutrophils and macrophages, which are key players in the innate immune response. In addition, we observed a rapid expansion of the B cell population upon infection, with B cells undergoing antigen-specific activation and maturation, suggesting a critical role during infection.
-
Cell Types Infected by H18N11: Immune cells, particularly macrophages, are primary targets of H18N11. Single-cell reconstructions of macrophages in tissues from infected animals using H18N11-specific genomic probes showed that these cells are productively infected.
-
Role of NS1 Protein: The NS1 protein of H18N11 appears to be critical for efficient viral replication in vivo, and its deletion impairs viral replication. Interestingly, the deletion of NS1 results in similar transcriptional changes compared to WT H18N11.
- Zoonotic Potential: H18N11 can infect human leukocyte subpopulations and replicates efficiently in PBMC-derived human macrophages. Therefore, zoonotic potential cannot be excluded.
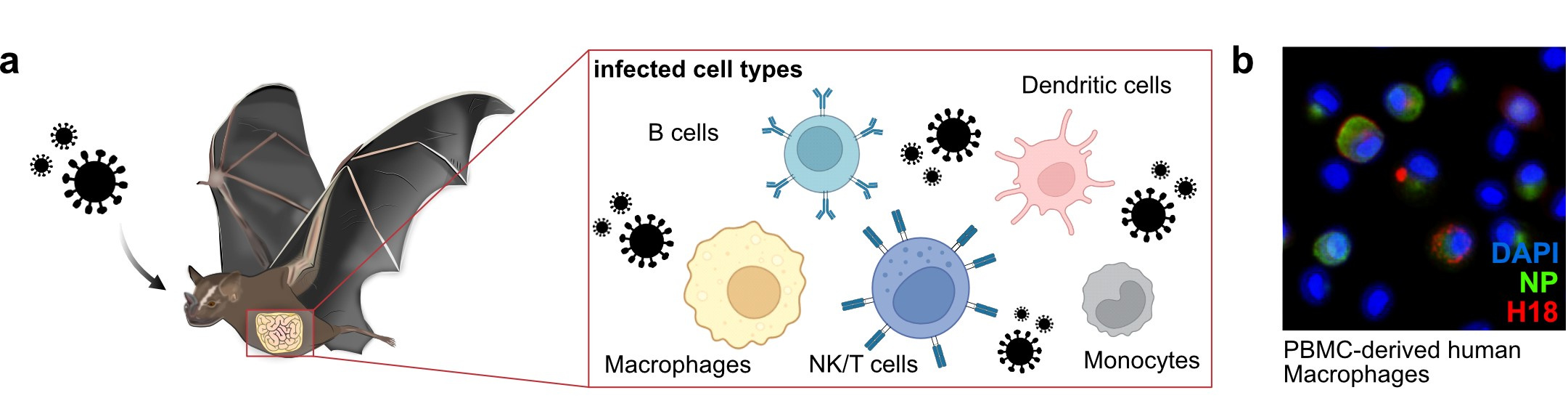
Why is this study important?
Bats are reservoirs for many viruses, some of which can jump to humans and cause severe disease while remaining inconspicuous in bats. Understanding bat-specific immune responses and how bats cope with these viruses will help us to better understand virus-host dynamics. This knowledge provides a basis for predicting the zoonotic potential of viruses and can aid in prevention.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in