Deeper insight into elementary processes of atmospheric gas-phase reactions
Published in Chemistry
Biogenic and anthropogenic emissions are released into the Earth´s atmosphere with an annual strength in the order of 109 metric tons of carbon. Their oxidation products can play an important role for human´s health as well as for the radiative forcing of the atmosphere. The detailed understanding of individual oxidation pathways is still a challenging task. For example, in the last years it has been discovered that isomerization steps of peroxy (RO2) radicals, which represent the key intermediates in the course of atmospheric oxidation, can outrun the “traditional” bimolecular RO2 radical reactions with NO or HO2 radicals, resulting in a distinctly changed product distribution.
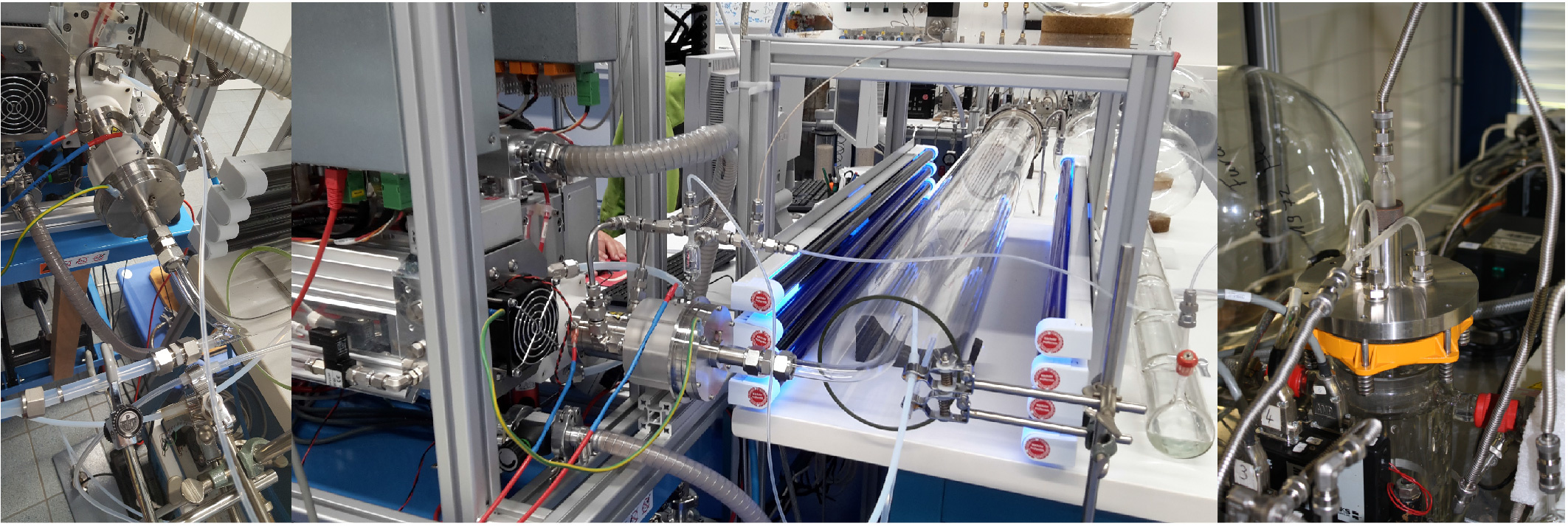
For our laboratory measurements, we are using a free-jet flow system that allows for the investigation of the early stages of an oxidation reaction for atmospheric conditions in the absence of interfering wall effects. This flow system has been developed in collaboration with process engineers, in order to overcome wall loss effects and potential heterogeneous wall reactions, especially of reactive intermediates such as Criegee intermediates and RO2 radicals.
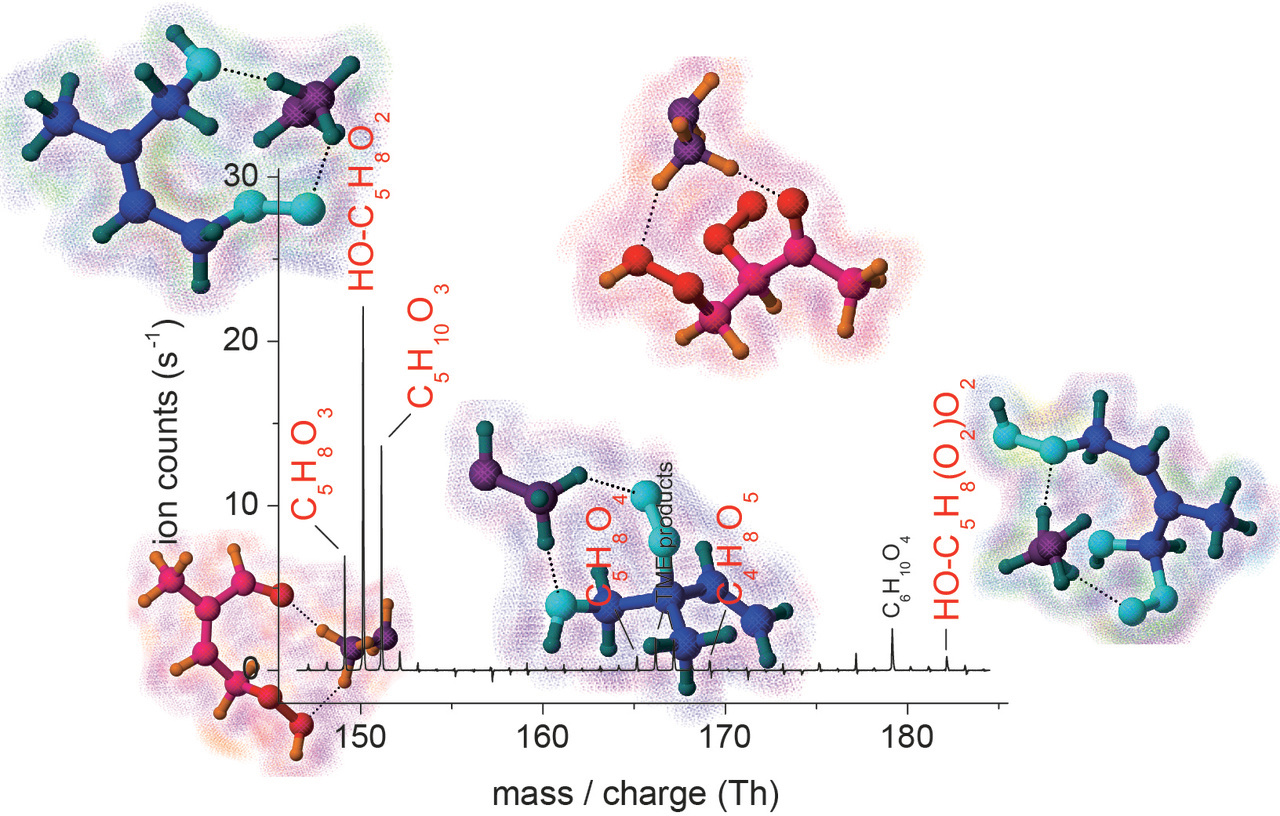
Direct monitoring of the intermediates and resulting closed-shell products formed in the experiments became possible using chemical ionization mass spectrometry applying a set of different reagent ions. Beside the well-established ionization by means of acetate, CH3COO-, or iodide, I-, recently the utilization of protonated amines and protonated hydrazine has been discovered as an efficient approach for sensitive detection of less oxidized compounds. The development of the ionization schemes is supported by quantum chemical calculations on the bonding between the reagent ions and sample molecules, which provide further information about the effectiveness of the different reagent ions in chemical ionization. Achieved detection limits for oxidized compounds are as low as 103 - 104 molecules cm-3, i.e. clearly lower than ppq level (ppq: part-per-quadrillion, 1 / 1015).
Reaction conditions can be chosen in such a way that solely the formation of primarily formed RO2 radicals and their unimolecular pathways can be studied. For rising RO2 radical concentrations, RO2 radical self- and cross-reactions become more important and their product channels are detectable. Addition of a second reactant, such as NO or HO2 radicals, allows for the following of the product formation from other selected bimolecular RO2 radical reactions. All in all, a more direct insight into RO2 radical processes during the initial stages of a reaction is possible using this technique.
See what´s possible investigating the early product channels of the OH + isoprene reaction for low NO and low HO2 radical conditions, and read the full story here: Berndt, T., Hyttinen, N., Herrmann, H. & Hansel, A. First oxidation products from the reaction of hydroxyl radicals with isoprene for pristine environmental conditions. Communications Chemistry 2, 21 (2019).
https://doi.org/10.1038/s42004-019-0120-9
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in