When Cancers Outsmart the Immune System from the Very Beginning
Published in Biomedical Research
The puzzle of immune evasion
Colorectal cancers are peculiar. They often show clear signs that immune cells are present — and in many patients, the number of tumour-infiltrating lymphocytes can even predict outcomes. Yet, therapies that “reawaken” the immune system, such as immune checkpoint inhibitors, are surprisingly effective in only a small fraction of colorectal cancers.
This mismatch puzzled us. If immune cells are present, why are they failing to eliminate the tumour? And if drugs that unleash the immune response don’t always work, what else is happening beneath the surface?
Looking deeper with multi-region, multi-omic tools
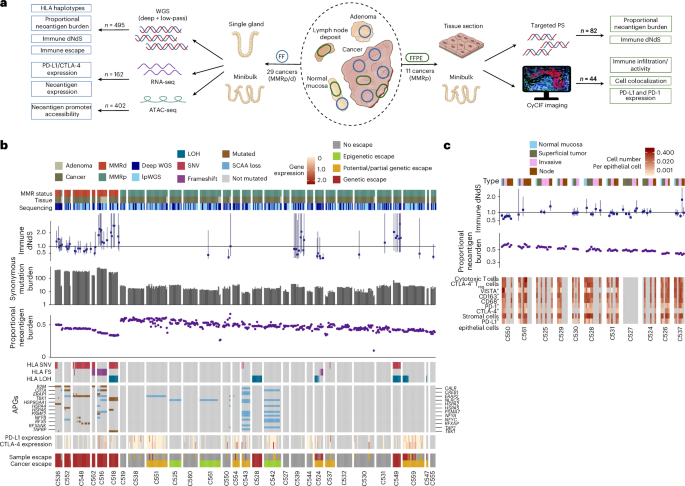
To answer these questions, we took a new approach. Rather than studying tumours in bulk, which can blur the picture, we zoomed in at single-gland resolution — analysing almost 500 microdissected glands from 29 colorectal cancers, alongside 82 carefully profiled micro-biopsies.
This gave us a unique opportunity to map, in high definition, how genetic mutations, epigenetic changes (alterations to chromatin structure that control gene expression), and the tumour microenvironment all interact with the immune system.
Our aim was ambitious: to capture the evolutionary timeline of immune evasion in colorectal cancer.
A surprising “Big Bang”
We found that, instead of a gradual accumulation of immune escape mechanisms, most tumours appeared to acquire their “cloaking devices” very early — essentially at the moment they transformed from benign adenomas into invasive cancers.
This is what we call a “Big Bang” model of immune evasion. Just as previous work from our group has shown that the growth of colorectal cancer itself follows a Big Bang dynamic — with early mutations setting the trajectory for the entire tumour — here we found that immune escape also happens explosively, at the very start.
In other words: by the time a cancer is recognisable, it has already armed itself against the immune system.
Epigenetics as a stealth strategy
One of the most striking findings came from the epigenome. We observed widespread changes in chromatin accessibility — essentially, the opening and closing of regions of DNA that determine which genes can be expressed.
Crucially, many of these changes occurred in the promoters of genes responsible for presenting neoantigens (the abnormal protein fragments that alert the immune system to cancer). In most cases, the effect was to “close” these promoters, silencing the very signals that could have revealed the tumour to T cells.
This is an elegant form of stealth: instead of mutating the genes themselves, tumours rewire the way those genes are switched on or off. It’s like turning off the lights in a house so a passing patrol won’t notice movement inside.
Genetic escape routes too
Of course, cancer doesn’t rely on epigenetics alone. We also saw clear evidence of genetic immune escape — such as mutations in HLA genes (the molecules that display antigens to immune cells) and loss of beta-2 microglobulin, which is essential for antigen presentation.
What was most telling, however, was the timing: these high-impact alterations were often clonal, meaning they were shared across the entire tumour. That strongly suggests they were acquired early, before the tumour spread or diversified.
Meanwhile, smaller, low-impact mutations tended to appear later and remained confined to specific regions of the tumour.
The microenvironment plays its part
Our analysis of the tumour microenvironment added another layer to the picture. Across the cancers, we saw that cytotoxic T cells — the immune system’s frontline soldiers — were present but kept at a distance from tumour cells. Regulatory T cells, which suppress immune responses, were enriched in tumour regions.
Interestingly, the invasive margin — where the tumour meets healthy tissue — seemed to be a hotspot for ongoing skirmishes. Here, we saw higher levels of immune surveillance and localised signs of immunoediting, as if small tumour subclones were still being tested by the immune system. But these were minor battles compared to the decisive early war the tumour had already won.
What this means for immunotherapy
Our findings have sobering implications. If immune evasion is “baked in” from the earliest stages of cancer development, then waiting until tumours are established may be too late for many patients. Immunotherapies might fail not because they don’t work, but because the tumour has already silenced the very signals that would make them effective.
On the other hand, our work also points to new opportunities. Because many escape mechanisms are epigenetic, they may be reversible. Drugs that open up chromatin or reactivate silenced genes could, in theory, restore antigen presentation and make tumours visible again to the immune system.
It also suggests that the best time to intervene may be much earlier, before immune escape is fixed. This raises the tantalising possibility of preventive immunotherapy in high-risk patients, or early interventions during pre-cancerous stages.
A new view of cancer-immune evolution
Stepping back, our study reframes how we think about the relationship between cancer and the immune system. Rather than a constant back-and-forth battle, it may be more like a sudden coup: a tumour that manages to disable immune recognition right at the start can grow largely unchallenged, shaping the battlefield in its favour.
This doesn’t mean the immune system stops trying. At the invasive margin, we still see signs of active skirmishes. But the larger war has already been decided.
Looking ahead
For us as researchers, this is both exciting and daunting. It means we need to rethink strategies for cancer immunotherapy, taking into account not just genetics but also the epigenetic and microenvironmental layers that cancers exploit.
It also opens the door to new collaborations: between cancer biologists and epigeneticists, between histopathologists and evolutionary modelers, between clinicians and data scientists. Only by weaving these perspectives together can we hope to stay a step ahead in this evolutionary race.
In the end, our study underscores one thing above all: timing matters. In colorectal cancer, the immune system’s chance to eliminate the tumour may come — and go — in the blink of an eye. Understanding that window could be the key to transforming patient outcomes.
Follow the Topic
-
Nature Genetics

This journal publishes the very highest quality research in genetics, encompassing genetic and functional genomic studies on human and plant traits and on other model organisms.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in