Delineating the differential phenotypic readouts of novel and canonical colorectal cancer NRAS and KRAS mutants
Published in Cancer

Rare, novel mutations in a heterogeneous tumor are often dismissed as inconsequential especially if the primary tumor can be surgically removed. This presupposes, however, that tumors have not spread to potentially inaccessible sites 1. If they have, intra-tumoral heterogeneity becomes relevant and may be linked to degree of metastatic dissemination and clinical progression 2. In recent years, the usefulness of identifying uncharted mutations, as well as their significance in de novo and acquired modes of resistance to targeted therapies have also gained better appreciation. In colorectal cancer (CRC), this is particularly relevant, given that patients refractory to anti-EGFR therapy continue to be identified, even if they are wild type in mutational hotspots of EGFR pathway genes. Consequently, learned cancer societies continually update their guidelines to include identification of these additional negative predictive biomarkers. Among these are non-hotspot mutations in KRAS, NRAS, BRAF, PIK3CA and PTEN.
NRAS is mutated in 2–5% of CRCs 3,4. In several studies, patients harboring mutations in NRAS were shown to be unresponsive to anti-EGFR therapy 5, prompting the European Medicines Agency to discourage the use of anti-EGFR drugs for these patients. While the phenotypic readout of KRAS mutants are well defined, however, NRAS mutations remain largely uncharacterized, including those in analogous codon positions the isoform shares with KRAS. Although less studied functionally and mechanistically, NRAS mutations have been associated with worse prognosis and overall survival compared to metastatic CRC driven by KRAS mutation or dysregulation 6. Patients with NRAS mutations and are wild type for KRAS also respond poorly to the anti-EGFR cetuximab, when compared to NRAS wild type carriers. Indeed, the American Society for Clinical Oncology was correct in suggesting to go beyond KRAS exon 2 during pathologic evaluation 7,8, which should include other downstream effectors of the EGFR pathway and the NRAS isoform. More importantly, the functional and phenotypic characterization of these rare but relevant mutations is important to determine the differential oncogenic potencies of these mutations which may have repercussions in the choice of therapeutic interventions. Already, characterization of isoform- and exon-specific mutations has proven to be instructive in the case of NRAS. In the study by Schirripa et al 5, overall survival seemed to depend on the position of the mutation in exons, with survival being shorter in those with exon 3 mutations compared to those who are wild type or have exon 2 mutations.
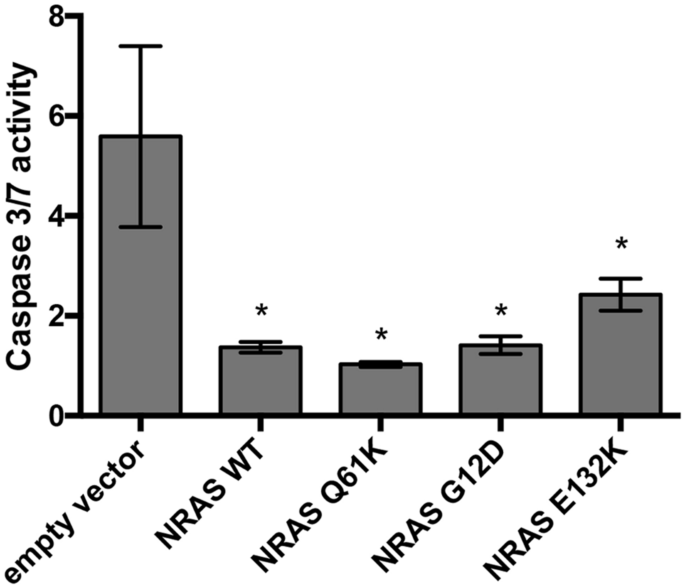
Our study investigated the phenotypic readouts of the novel NRAS mutant E132K identified in three young-onset sporadic colorectal cancer patients, as well as the poorly characterized canonical mutants NRAS G12D and NRAS Q61K. The mutants show partially overlapping but mostly distinct oncogenic phenotypes when compared to KRAS mutants including those with mutations in the analogous codons 12 and 61. They do not affect cellular proliferation and motility, but show resistance to apoptosis, cytoskeletal reorganization, and loss of adhesion. However, while RAS isoform-specific oncogenic mutant effects were delineated in the study, the nuanced effects of exon-specific mutations in NRAS need to be further resolved, including their differential downstream effector engagement. Further analyses of young-onset versus late-onset CRC datasets are also necessary to qualify NRAS E132K as a biomarker for the young-onset subtype.
Link to the paper: https://www.nature.com/articles/s41598-020-67796-8.pdf
- Vogelstein, B. et al. Cancer Genome Landscapes. Science 339, 1546–1558 (2013).
- Iacobuzio-Donahue, C. A., Litchfield, K. & Swanton, C. Intratumor heterogeneity reflects clinical disease course. Nat Cancer 1, 3–6 (2020).
- Irahara, N. et al. NRAS mutations are rare in colorectal cancer. Diagnostic Molecular Pathology 19, 157–163 (2010).
- Palomba, G. et al. Prognostic impact of KRAS, NRAS, BRAF, and PIK3CA mutations in primary colorectal carcinomas: A population-based study. Journal of Translational Medicine 14, 1–11 (2016).
- Schirripa, M. et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. International Journal of Cancer 136, 83–90 (2015).
- Cercek, A. et al. Clinical Features and Outcomes of Patients with Colorectal Cancers Harboring NRAS Mutations. Clinical Cancer Research 23, 4753–4760 (2017).
- Helwick, Caroline. Time to Think Beyond KRAS in Metastatic Colorectal Cancer. https://www.ascopost.com/issues/december-1-2013/time-to-think-beyond-kras-in-metastatic-colorectal-cancer/.
- Katz, Julie; DuBell, Arnold. Beyond KRAS exon 2: Research at ASCO and Important Treatment Implications for Metastatic Colorectal Cancer.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in