
Alloy development typically employs two types of practices to achieve the desired properties. One is intermixing of multiple mixable components to form a solid solution, termed as alloying. The other is the demixing of the alloying components, leaving behind an altered residual structure, termed as dealloying. In the early 1900s, Hume-Rothery set some simple yet effective rules to predict if two elements can form a solid solution based on the intrinsic parameters such as atomic size, crystal structure, electronegativity, and valences of the elements. Since then, the Hume-Rothery rules have earned great reputation over the past century in the field of materials science in designing new alloys. However, it has also been shown more recently that the Hume-Rothery rules do not hold at the nanometer scale 1 or under specific conditions 2. These findings demonstrate the breakdown of the Hume-Rothery rules by mixing unmixable elements.
The other half of the situation regarding the Hume-Rothery rules is yet untouched. That is, does self-demixing occur in solid solution alloys occur without the assistance from a chemical dissolution process? Our motivation for this research is therefore to test the possibility of self-demixing alloying elements in solid solution alloys. The Cu-Au alloy is an ideal model system to investigate the demixing behavior of the miscible systems because it fulfills the Hume-Rothery Rules and forms a face-centered cubic (FCC) solid solution through the full range of composition and temperature. Indeed, we observed unexpected self-dealloying in the Cu-Au alloy through a phase separation process that differs from the chemical dealloying mechanism and thus represent a departure from the Hume-Rothery Rules. At the elevated temperature of experiments, the surface of the Cu-Au solution has many active sources including atomic steps, ledges and kinks for the massive formation of Cu and Au adatoms via step edge detachment (Fig. 1). The disparity in the adatom–substrate exchange barriers separate Cu adatoms from the mixture of Cu and Au adatoms, leaving behind a fluid phase enriched with Au adatoms that subsequently aggregate into supported clusters that exhibit a rich variety of dynamics including grain rotation and structural oscillations dynamics resulting from the cluster and support interfacial interactions. Through the use of real-time transmission electron microscopy (TEM) observations and atomistic simulations, we further delineate the atomic-scale mechanisms underlying the nucleation, rotation and amorphization-crystallization oscillations of the Au clusters (Fig. 2). We envision the broader applicability of this process because size differences between constituent atoms in multicomponent materials can typically lead to the different adatom–substrate exchange barriers between dissimilar atoms, thereby inducing the phase separation in multicomponent materials. The observed phenomena are of considerable practical importance as the phase separation process has wide relevance for a broad range of material systems, properties, and reactions, which include metallurgy, nanostructure synthesis, and heterogeneous catalysis.
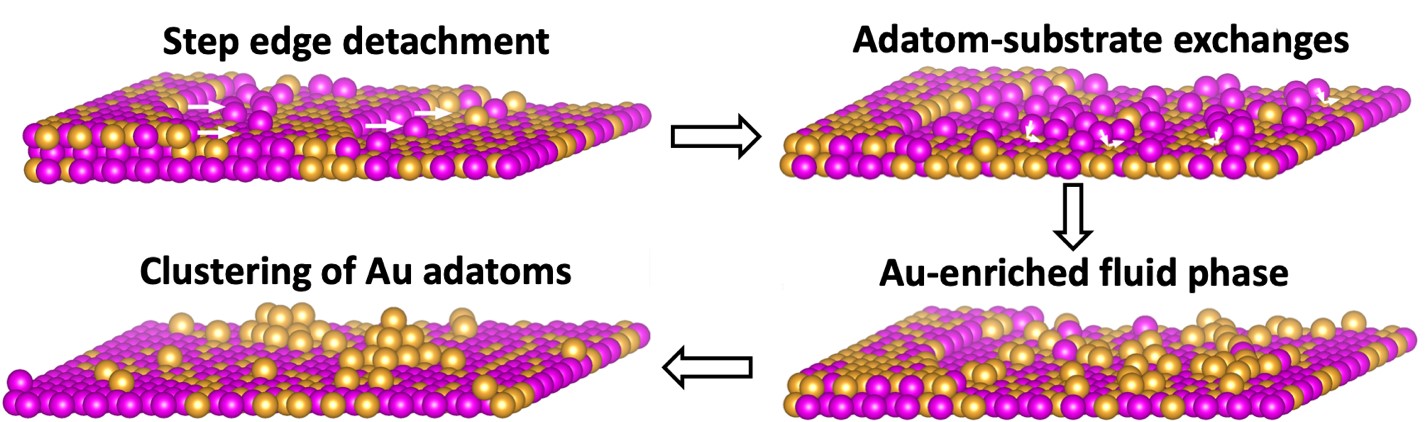
Figure 1. Schematic illustrating the phase separation process from the formation of a Cu-Au fluid phase of Cu and Au adatoms by step-edge detachments to the adatom-substrate exchange induced enrichment of Au atoms in the fluid phase and then to the clustering of Au adatoms.
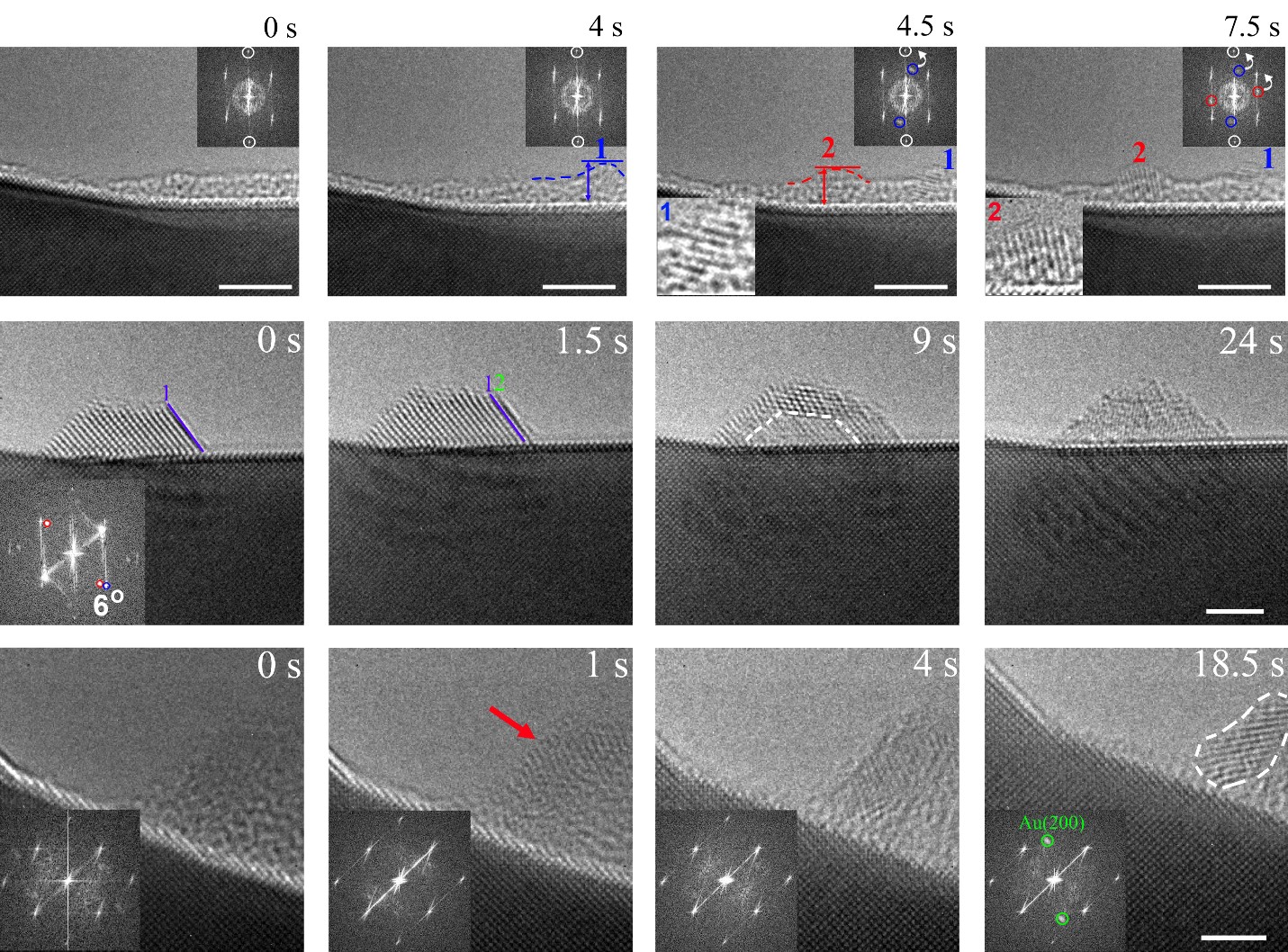
Figure 2. In-situ TEM observations of the nucleation, growth, and structural oscillation of Au clusters.
These results were recently published in Nature Communications:
https://www.nature.com/articles/s41467-020-17826-w
References:
- Miyajima K, Fukushima N, Himeno H, Yamada A, Mafuné F. Breakdown of the Hume− Rothery Rules in Sub-Nanometer-Sized Ta-Containing Bimetallic Small Clusters. The Journal of Physical Chemistry A 113, 13448-13450 (2009).
- Desgreniers S, John ST, Matsuoka T, Ohishi Y, Justin JT. Mixing unmixables: Unexpected formation of Li-Cs alloys at low pressure. Science Advances 1, e1500669 (2015).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in