Distinguishing the Indistinguishable, Making Water out of Wine, and Other Quests

One of the basic tenets of modern science is that one atom of an element is like any other atom of that same element. So how do you tell them apart? The question becomes important when examining how hydrogen atoms move in a molecule. Our recent project, Initial-site characterization of hydrogen migration following strong-field double ionization of ethanol, published earlier this month in Nature Communications, explored hydrogen migration in the dissociation of ethanol (CH3CH2OH) dications, i.e., hydrogen atoms (or protons) moving from their site of origin to the final hydrogen-rich fragment. The goal was to determine the origin site for each hydrogen included in a hydrogen-rich fragment and the associated probability for each initial site combination of hydrogens. Clearly, this implies that the different hydrogen sites had to be distinguished.
For example, when tri-hydrogen ion (H3+) is the final product, we wanted to determine from which site these atoms came and evaluate the probability of each initial site contribution using the measured data. Ekanayake et al. [1] suggested that H3+ can be formed by the elimination of an H2 molecule, consisting of both hydrogen atoms attached to the central carbon, which then roams around and abstracts a proton either from the hydroxyl (OH) group or the methyl (CH3) group [see figure 1(a)]. Our goal was to quantify this interpretation and provide the likelihood for each of these two processes through their initial site combinations.
Deuterium tagging, i.e., the substitution of all the hydrogen atoms in a specific site(s) by deuterium isotopes, has been used successfully in smaller molecules. In ethanol, however, there are three sites for only two hydrogen isotopes. This problem can be overcome, in principle, by repeating the same measurements using different isotopologues, i.e., different deuterium-hydrogen combinations. There are eight permutations for replacing one hydrogen site in ethanol with deuterium [see Figure 1(b)], and we could find seven of them commercially.
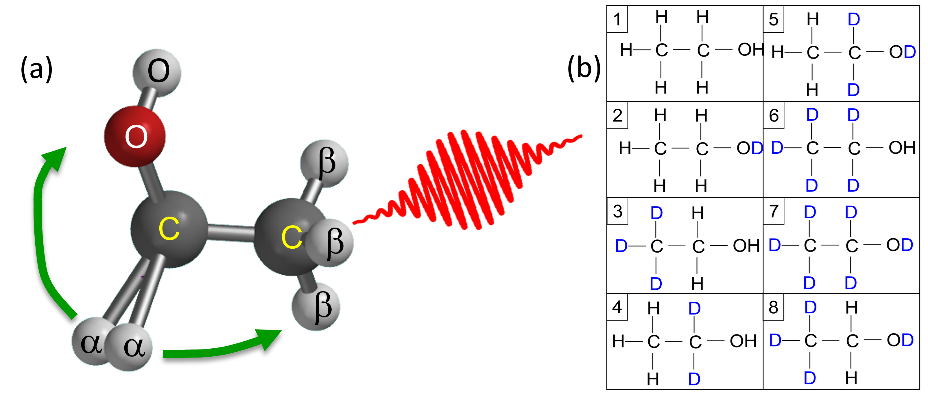
Fig. 1. Ethanol cartoons of (a) structure, site definitions (alpha, beta, and O), and roaming paths, (b) the different isotopologues used in the experiment.
So far, the plan sounds good. But the task gets more challenging from this point. The experiments are done using “COLTRIMS” (COLd Target Recoil Ion Momentum Spectroscopy), which yields the three-dimensional momentum vectors of all the ionic molecular fragments produced in a laser-molecule interaction. The data is built up laser pulse by laser pulse, with each event adding to the repository of data that can be analyzed afterward, similar in principle to many nuclear or particle physics experiments. Eventually, provided there are sufficient statistics, this data can be used to build multi-dimensional histograms that describe the probability of a process.
H3+ formation is not the primary process in these events, so the data acquisition time is long, and the experiment (laser, molecular beam, electronics, vacuum systems) must be kept stable for the duration of the measurement. Then, the process had to be repeated six more times! In the article, this Herculean task is distilled into a single phrase, “Keeping the experimental conditions the same across all measurements was a point of emphasis…” The hero behind this “point of emphasis” was then-graduate student Travis Severt, who built a detailed scheme to monitor all experimental conditions and assembled a team from several groups at Kansas State University to carry it out. The collegial and collaborative nature of the J.R. Macdonald Laboratory was in full effect during the weeks of data collection this tour de force experiment required.
Fig. 2: Dr. Travis Severt in the J.R. Macdonald Laboratory at Kansas State University.
The result of this remarkable experimental work was a mountain of data. Beyond an initial survey [1], the route to the top of the mountain was unclear, to the point that none of us were sure it even existed. Travis was deeply involved in other work [2,3] and was completing his Ph.D. studies [4], so another hero was needed to take up the quest.
In good literary fashion, the new hero appeared from the north, in the form of second-year Augustana University undergraduate Eleanor Weckwerth. Via numerous ZOOM conferences, Travis trained Eleanor on the analysis code he had developed, and she set to work establishing a trail to the top of Mt. Ethanol.
To make the comparisons between isotopologues of ethanol, a reference standard was needed, and the logical one is a branching ratio, defined as the yield of a specific final product relative to the total yield. Thus, Eleanor had to analyze not just the dissociation channels of interest, but all the dissociation channels needed to define the total yield in the denominator of the branching ratio.

Fig. 3: Eleanor Weckwerth, now beginning graduate school at Stanford University.
Furthermore, when you write out all the possibilities, you find that the site-specific probabilities are overdetermined in the data. Eleanor’s innovative approach to this challenge was to incorporate all the possible formation routes, from all the different isotopologues, and use a fitting technique to find the optimal solution for all unknowns simultaneously. She also produced similar sets of equations for other hydrogen-rich fragments (H2O+, H3O+, and CH4+) from ethanol. The systems of equations occupy a significant amount of space in the Supplemental Information (one set includes 54 equations!). Each equation depends on at least one, and usually, multiple measured branching ratios that Eleanor painstakingly evaluated from the data. Finally, she added a Monte Carlo-based error analysis to quantitatively evaluate the uncertainty.
Eleanor went on to apply this approach to cases where the production of H3+ was accompanied by the loss of one or two hydrogen atoms. The result is a systematic, quantitative, assessment of how likely it is for a particular initial site to contribute to the formation of H3+ and the other molecular ions described in the article.
Making progress on this experiment was fulfilling because it was never clear that the challenges of each successive step were surmountable. Because of the innovation and hard work of Travis, Eleanor, and all our colleagues who worked on this project, the experimental goal was accomplished.
The larger quest is not over. The data we collected gives a fairly complete picture of the origin of the hydrogen atoms that form molecular ions such as H3+ during the dissociation of ethanol. We are at work uncovering the dynamics of some of these pathways. But the real challenge is now turned over to theoretical counterparts. Can a combination of high-level electronic structure calculations and molecular dynamics simulations of the subsequent dissociations explain our measured probabilities for specific initial site compositions? More heroes are needed.
References
- N. Ekanayake, et al. Nature Communications 9, 5186 (2018).
- J. Rajput et al., Phys. Rev. Lett. 120, 103001 (2018).
- T. Severt, et al., Nature Communications 13, 5146 (2022).
- T. Severt, PhD thesis, Kansas State University (2021).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor – Space Physics, Quantum Physics, Atomic, Molecular and Chemical Physics
Got a question for the editor about Space Physics, Quantum Physics, Atomic, Molecular and Chemical Physics? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in