DNA methylation at birth and attention problems in preterm children
Published in Genetics & Genomics, General & Internal Medicine, and Paediatrics, Reproductive Medicine & Geriatrics

Children born preterm, especially those born < 30 weeks gestational age (GA), are at risk of developing attention-deficit/hyperactivity disorder (ADHD). This common condition is characterized by difficulty paying attention, impulsive behavior, and hyperactivity. Attention problems can cause children to have challenges in social and academic settings. We currently lack the ability to identify which preterm infants will go on to develop ADHD, though early identification could help us provide additional support to children and families that might lessen the negative impacts of this condition. In the current study, we followed children born < 30 weeks GA to see if we could identify biological factors at birth that predicted differences in attention problems – a core symptom of ADHD – when children were 2 years old.
Why study attention problems in preterm infants?
ADHD is one of the most prevalent mental health conditions in childhood. It is 2 to 4 times more common among children born preterm. It is commonly diagnosed in childhood but can persist into adolescence and adulthood. ADHD can impact how people act at home, in school, and with friends. There are effective treatments for ADHD such as medication and therapy that can help alleviate symptoms, teach coping strategies, and promote healthy behavior. The earlier that treatment begins, the better the outcomes for children. While attention problems are common among preterm children, not everyone is affected. In order to provide better and earlier treatment, it is important to identify early on which preterm infants are at highest risk for attention problems.
How can epigenetics help us understand ADHD risk?
Decades of research have shown that ADHD risk is largely influenced by genetic differences, meaning differences in genetic makeup between people. Despite this, there are many other factors that are associated with ADHD risk. Factors in the environment, like diet, heavy metal and chemical exposures, and family adversity can all contribute to risk for ADHD. Recently, scientists have discovered that differences in gene activity (not just differences in genetic makeup) are associated with attention problems and ADHD. The term epigenetics describes the biological processes that can alter gene activity. We often talk about epigenetic processes turning genes ‘off’ or ‘on’, but it is more accurate to think of these processes operating like a dimmer switch that can ‘dim’ or ‘enhance’ the amount of gene expression. One of the most commonly studied epigenetic processes in humans is DNA methylation (for more information, see “Want to know more about DNA methylation?” below) Generally speaking, higher levels of DNA methylation are thought to lower levels of gene expression, especially when methylation occurs in certain areas of the gene known as promoter regions. By changing gene expression, DNA methylation can have wide-reaching biological and behavioral consequences. Both genetic and environmental factors can contribute to differences in DNA methylation between people.
DNA methylation and ADHD
The best evidence linking DNA methylation to ADHD comes from epigenome-wide association studies (EWAS). These studies test for differences in DNA methylation across many different locations in the genome (> 400-800K sites). These locations where DNA methylation occur are called CpG sites. Results from prior EWAS show associations between levels of DNA methylation at specific CpG sites and later ADHD diagnosis or symptom severity. While this is promising evidence, all of the prior studies have studied children born full-term, who differ in many ways from children born preterm.
What this study adds
This is the first study to specifically investigate epigenetic predictors of attention problems in children born very preterm (< 30 weeks GA). This group is important to study because they are at higher risk of ADHD. They may have differences compared to children born full-term, such as different rates of prenatal and postnatal medical complications, so the results of studies with full-term infants may not apply as well to them. We also studied attention problems in toddlerhood (at age 2) rather than later childhood, because of the importance of early identification of children who may be at high risk for downstream ADHD.
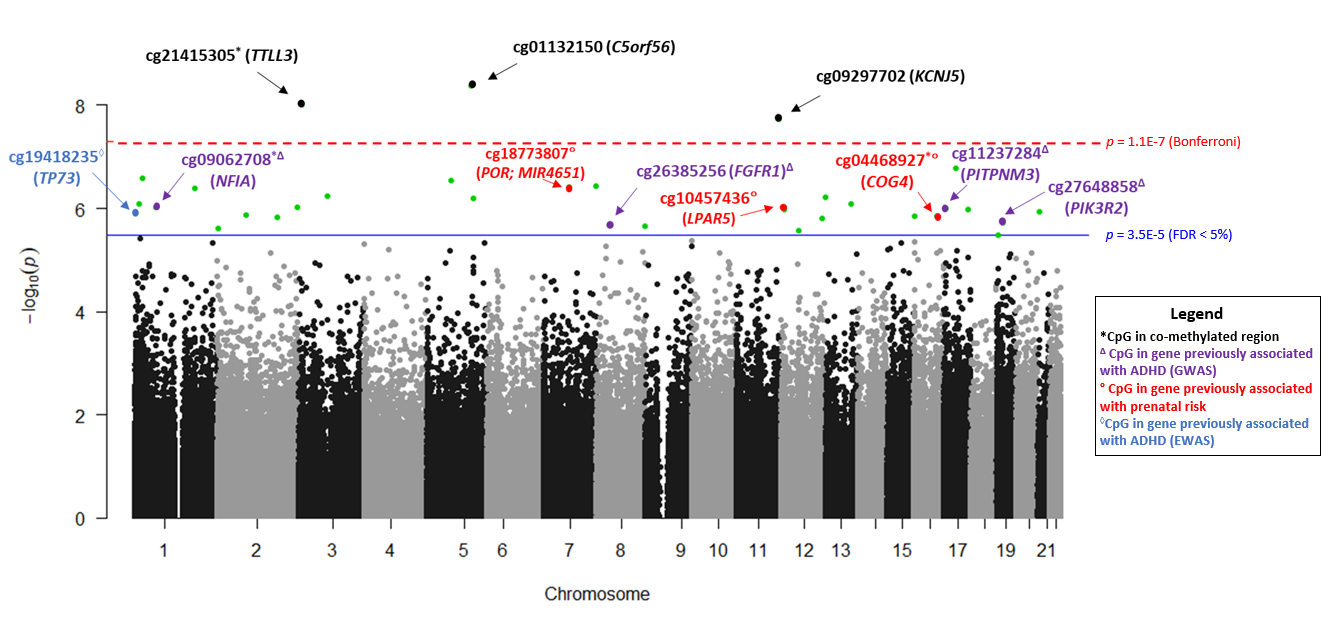
We studied 441 children born < 30 weeks GA who we have been following since birth. Children were recruited from 9 hospitals at 6 research sites across the United States, from 2014 to 2016. At the time they were discharged from the neonatal intensive care unit (NICU), we collected a cheek swab that we tested for levels of DNA methylation at over 450,000 sites across the genome. When children were 2 years old, they came back for a follow-up visit and parents were asked to rate their child’s level of attention problems using a well-known behavior questionnaire. We found that DNA methylation at 33 CpG sites were associated with child attention problems. Of these, 6 CpGs were positively associated (higher methylation associated with more attention problems) and 27 were negatively associated (lower methylation associated with more attention problems). Several of the CpGs we identified were located in genes that have previously been linked to ADHD.
Conclusion
By measuring DNA methylation at birth, we may be able to better predict which preterm children will go on to develop attention problems and perhaps even an eventual ADHD diagnosis. This information could help us identify preterm children and their families who could benefit from early intervention, with the goal of promoting positive health and behavioral outcomes. In the future, it will be important to study how genetics, epigenetics, and the environment work together to influence risk for ADHD in children.
Want to know more about DNA methylation?
To better understand DNA methylation, we first need to review DNA itself. The cell nucleus contains chromosomes, which are made up of tightly coiled strands of DNA that contain genes. The sequence (or ‘code’) of a gene is made up of a sequence of 4 base pairs: adenine (A), guanine (G), cytosine (C), and thymine (T). The sequence of DNA might look like this: GGCGATACAT. DNA code is ‘executed’ when it is transcribed into RNA, which is then translated into proteins. It is these proteins that ultimately determine the gene’s biological function.
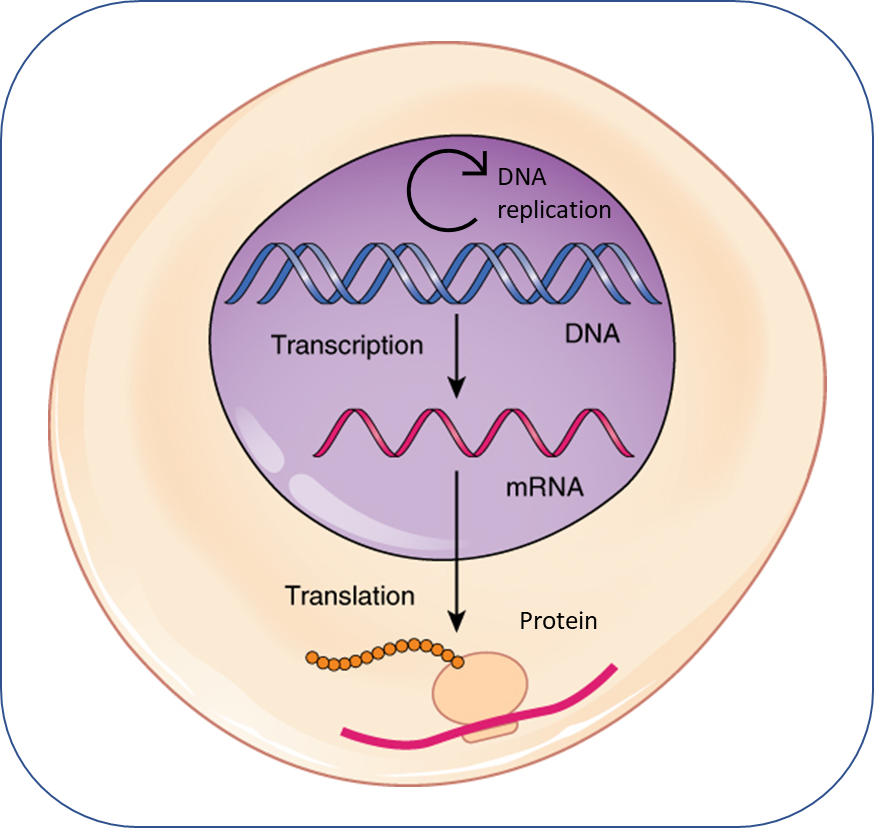
DNA methylation alters gene activity by changing the rate of transcription, ultimately changing the amount of protein produced. In this way, DNA methylation changes gene activity without changing the genetic code.
DNA methylation is a process by which a methyl group (CH3) is added to a portion of the DNA sequence where a cytosine (C) is followed by a guanine (G), linked by a phosphate group (p). This is termed a CpG site. In the sequence of DNA described above, the CpG site is located here: GGCGATACAT. Because the methyl group is added ‘on top of’ the DNA, it does not change the underlying genetic code (the sequence of A, G, C, and T). But, the presence of the methyl group can change the likelihood that the portion of genetic code will be expressed, by changing the rate of transcription. Changes in the rate of transcription ultimately change the amount of protein a gene produces. Thus, differences in DNA methylation between people (even people with identical underlying DNA sequence, like identical twins) can lead to differences in their biological and behavioral functioning.
For further reading, see: Lester, B. M., Conradt, E., & Marsit, C. (2016). Introduction to the Special Section on Epigenetics. Child Development, 87(1), 29–37. https://doi.org/10.1111/cdev.12489
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in