"Dragon III" Research for Gastric Cancer
Published in Cancer

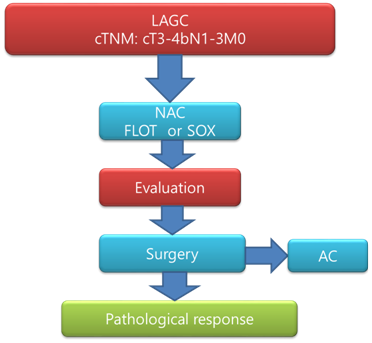
Neoadjuvant chemotherapy with docetaxel, oxaliplatin, fluorouracil, and leucovorin (FLOT regimen) has shown promising results in terms of pathological response and survival rate in patients with resectable locally advanced gastric cancer (LAGC). However, tegafur gimeracil oteracil potassium capsule (S-1) plus oxaliplatin (SOX regimen) is the preferred chemotherapy regimen in Eastern countries. Here, we conduct an open-label, two-arm, phase II randomized interventional clinical trial (Dragon III; ClinicalTrials.gov: NCT03636893) to evaluate the safety and efficacy of both regimens.
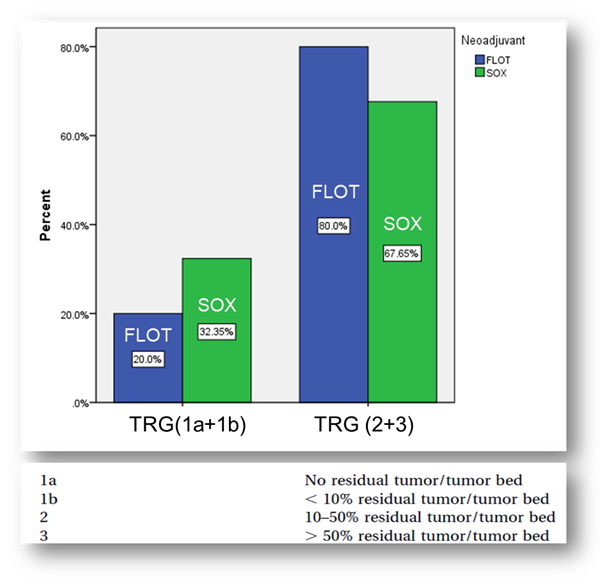
In this work, we compare the rate of postoperative tumor regression between the neoadjuvant chemotherapy FLOT and SOX groups. Our study does not find statistically significant differences between neoadjuvant FLOT and SOX regimens for the primary outcomes reported here in resectable locally advanced gastric cancer.
The idea for this study was simply induced by the recent publications on neoadjuvant chemotherapy for gastric cancer, especially on the FLOT regimen. As gastric cancer is one of the leading malignant diseases in East Asian countries, there is a need to conduct scientific research in the region. As the FLOT regimen was introduced in Germany, we did thorough investigations before going for this result. We conducted feasibility research on the FLOT regimen. Then we conducted this Phase II research to obtain the preliminary results and to explore whether it is necessary to undergo a Phase III study. The results of this current study urge a further large scale multicenter Randomized Controlled Trial.
The word "Dragon" is the simple translation of "Long" in Eastern literature, which is completely different from the word "Dragon" in western literature. “Long" in the eastern culture, is generally considered as a symbol of an auspicious creature which prevails over evil. We nominated the word "Long or Dragon" for series of clinical researches on Gastric Cancer to give the impression that the "Long or Dragon" is dedicated to the research on the treatment of this malignant disease that aims to cure gastric cancer. Currently, five different types of research are undergoing with different serial numbers under "Dragon". The "Dragon III" is a randomized controlled clinical trial on gastric cancer which compares the neoadjuvant FLOT with the SOX chemotherapy regimen for gastric cancer.

The initiator and principal investigator of all these “Dragon” or “Long” series clinical researches is Professor Zhenggang Zhu. Prof. Zhu is a famous scholar in the field of gastric cancer research in China, he has long served as the Chairman or Honorary Chairman of the Chinese Gastric Cancer Association, and he is also China’s representative of the Council for International Gastric Cancer Association. He spent about half a century in medical science. He is interested in both the clinical and basic researches to fight against gastric cancer and still enthusiastically active in this field. Prof. Zhu and his team work at Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine.

Prof. Zhu is the mentor of Dr. Birendra Kumar Sah. Dr. Sah originally hails from Nepal and he is very much interested in clinical researches on gastric cancer. He has been residing in Shanghai for more than 20 years.

Please visit below to read the full article
https://www.nature.com/articles/s41467-020-19965-6
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in