Electrochemical Synthesis of Circulenes
Published in Chemistry

1. The Chemistry of Polycyclic Heteroaromatics:
The chemistry of heteroaromatic compounds has been explored for about two hundred years, growing to include key areas such as synthetic and physical organic chemistry, natural products, molecular biology, and materials science. Among them, polycyclic heteroaromatic compounds (PHAs) have attracted special interest due to their unique combination of structural complexity and tunable electronic properties. With rigid π-conjugated backbones and adjustable redox and optical behavior, PHAs have become important in organic electronics, catalysis, molecular recognition, and chiral technologies.
Within this group, hetero[7]helicenes, dehydrohetero[7]helicenes, and hetero[8]circulenes are especially interesting due to their unique shapes and wide range of potential applications.
However, developing efficient and versatile synthetic routes to these complex structures remains challenging due to several factors:
- Long, multi-step procedures
- Harsh reaction conditions
- Dependence on costly or toxic catalysts
- Poor tolerance for diverse functional groups
To overcome these challenges, greener, milder and more efficient synthetic approaches are needed with improving functional group compatibility.
2. A Green and Modular Vision: Electrochemical Synthesis
Electrochemistry provides a sustainable alternative by using electricity as a clean reagent, avoiding toxic metals and stoichiometric oxidants, and enabling precise control of redox reactions. This raises a key question:
Can electrochemistry provide a selective and efficient pathway to synthesize complex polycyclic aromatics?
Our strategy focused on:
- Sustainability: metal-free, oxidant-free reactions using electricity.
- Selective Activation: exploiting tunable electrochemical oxidation potentials.
- Chemoselectivity: guided by the Kočovský principle1, predicting selective oxidative heterocoupling when oxidation potentials differ by ~0.25 V.
- Modularity: adaptable routes compatible with diverse substrates for broad functional group tolerance.
3. Electrochemical Synthesis of Dehydrooxa[7]helicenes:
In 2022, we advanced this approach by synthesizing dehydrooxa[7]helicenes— molecules that are more rigid and have larger connected π-systems, which makes them more stable and gives better optical properties. This was achieved through oxidative heterocoupling of carefully selected building blocks, resulting in over 25 examples with an average yield of about 84% (Scheme 1)2.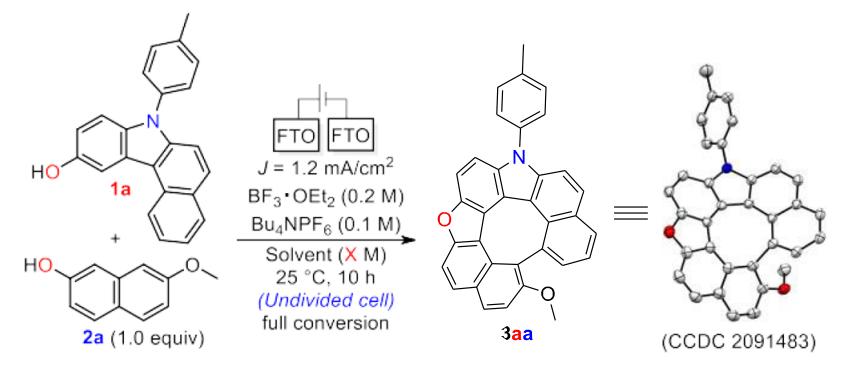
Scheme 1. Electrochemical Synthesis of Dehydrooxa[7]helicenes
4. Expansion to Oxaza[7]helicenes:
Building on previous success, in 2023 we expanded our electrochemical method to synthesize oxaza[7]helicenes—twisted molecules known for their unique optical and electronic properties. Using 3-hydroxybenzo[c]carbazole (1) and 2-naphthol (2) as examples, we took advantage of their different oxidation potentials to enable selective coupling and helicene formation. Without adding an electron-donating group at the 7-position of substrate 2, we stabilized intermediates and promoted the formation of oxa[7]helicenes, producing about 30 examples with yields up to 86%³.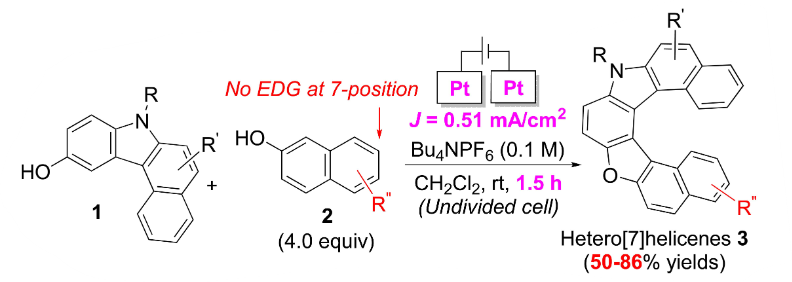
Scheme 2: Electrochemical synthesis of oxa[7]helicenes from 3-hydroxybenzo[c]carbazole and 2-naphthol derivatives.
After our previous results, we began to ask: Could electricity alone provide a direct and mild route to the synthetically challenging hetero[8]circulenes?
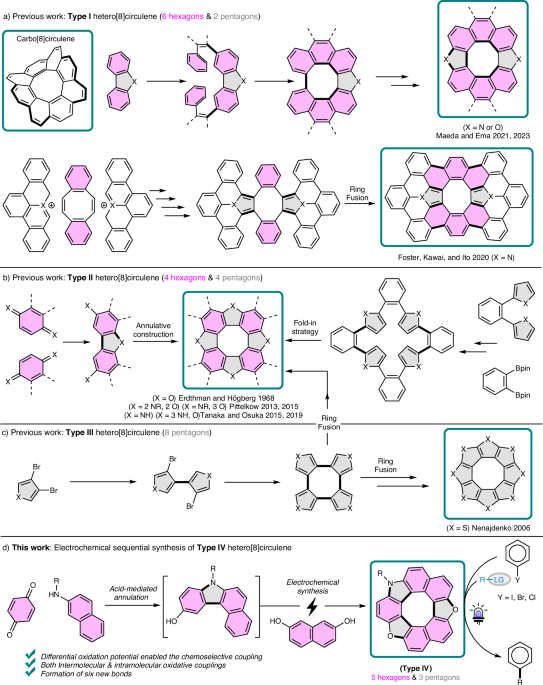
5. Hetero[8]circulenes:
The chemistry of hetero[8]circulenes dates back to the late 19th century, when von Knapp, Schultz, and Liebermann observed that mixing 1,4-naphthoquinones under acidic conditions formed insoluble oligomers. These were later identified by Erdthman and Högberg in 1968 as tetrameric structures.
Traditionally, hetero[8]circulenes are classified into three types based on ring composition:
- Type I: six hexagons and two pentagons
- Type II: four hexagons and four pentagons
- Type III: eight pentagons
6. Electrochemical Synthesis of Type IV Hetero[8]circulenes (this work):
In 2025, guided by the Kočovský criterion, we selected two suitable partners:
- 3-hydroxycarbazole (oxidation potential: 0.65 V vs. ferrocene)
- 2,7-dihydroxynaphthalene (oxidation potential: 0.92 V vs. ferrocene)
The 0.27 V difference in oxidation potential falls within the ideal range, enabling efficient and selective electrochemical cross-coupling to synthesize the novel Type IV hetero[8]circulenes (Scheme 3).
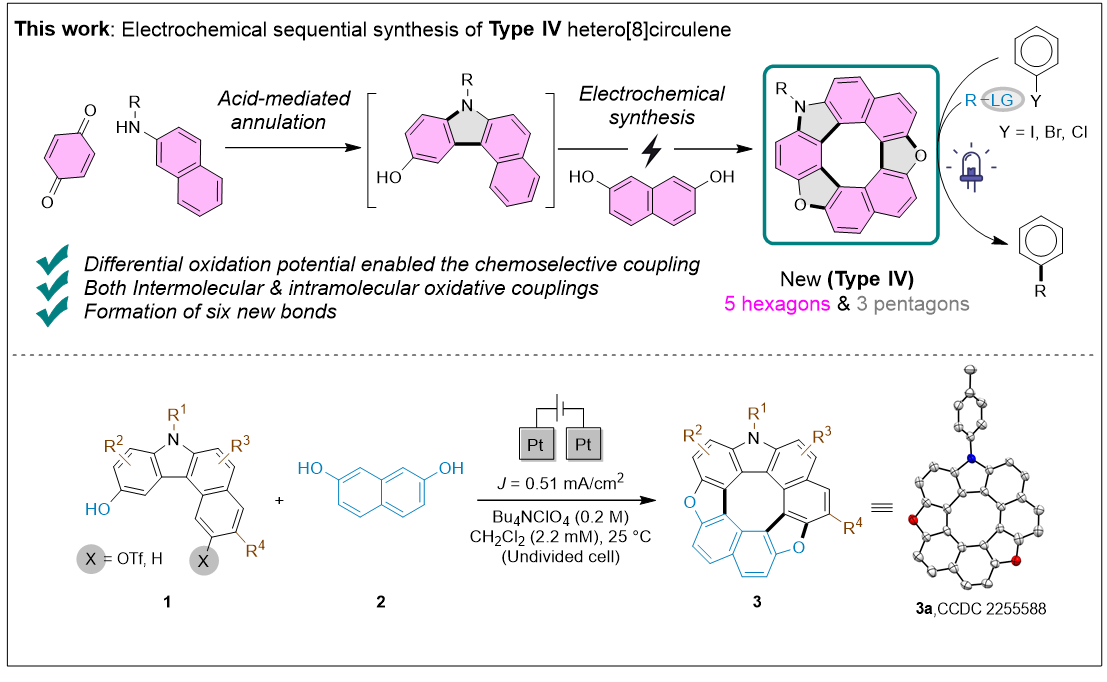
Scheme 3. Electrochemical synthesis of the novel Type IV hetero[8]circulene.
Starting with acid-mediated annulation of p-benzoquinones and 2-naphthylamine derivatives, followed by electrochemical oxidative coupling with 2,7-dihydroxynaphthalenes, we synthesized dioxaza[8]circulenes through selective inter- and intramolecular bond formation.
The metal-free, mild, proton-tolerant protocol yielded up to 83% in short times, producing 16 diverse, highly soluble hetero[8]circulenes with broad functional group tolerance. A one-pot protocol completed the synthesis in 15 hours with just one purification step, demonstrating scalability and ease of use. (Scheme 4).
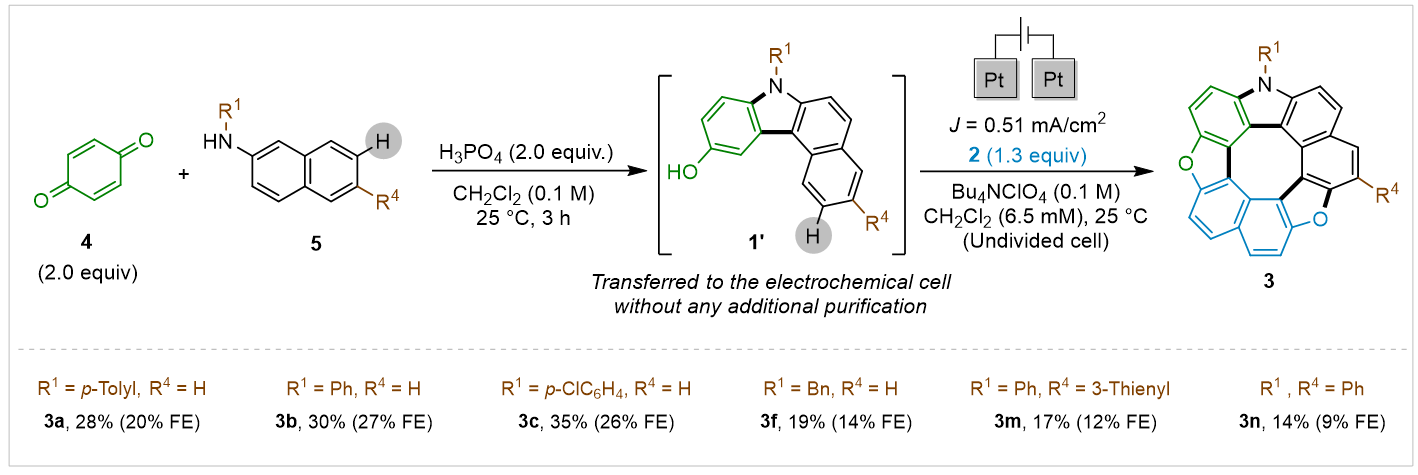
Scheme 4. One-pot Electrochemical synthesis of the novel Type IV hetero[8]circulene.
7. Structure, Properties, and Photocatalytic Activity
X-ray crystallography showed that dioxaza[8]circulene 3a has a planar structure with alternating bond patterns. Its outer rings are aromatic, while the center is paratropic, and in solid form, molecules stack closely through C–H···π interactions. It absorbs light at 389 nm and emits at 390 and 435 nm, with a 6.5% fluorescence quantum yield. Electrochemically, 3a exhibits three reversible reduction waves at −2.05, −2.14, and −2.46 V vs. SCE.
These features led us to test 3a as an organophotocatalyst. Its rigid structure, strong light absorption, and low reduction potentials made it ideal for photoredox reactions. Unlike metal-based systems, organic catalysts like 3a are cheaper, less toxic, and easier to handle.
Using 3a, we performed photoreduction of organic halides under 365 nm LED in DMSO with Cs₂CO₃ and 10 mol% catalyst at room temperature, yielding product 8a in 62% (Scheme 5).
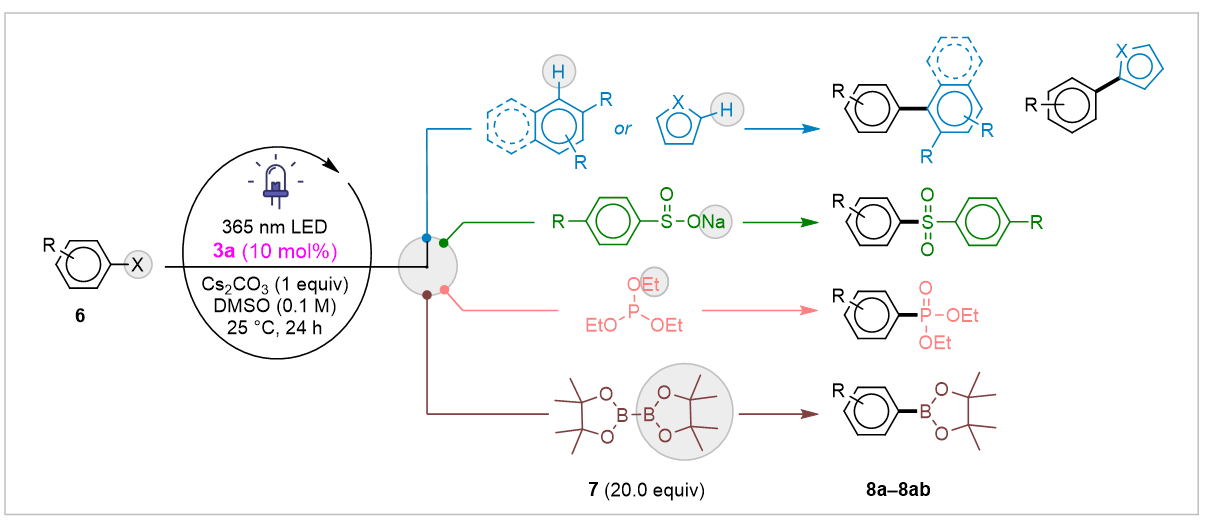
Scheme 5. Application of Hetero[8]circulene as an Organophotocatalyst for C–X Bond Formation.
Aryl iodides, bromides, and chlorides reacted as expected, with electron-rich arenes successfully cross-coupled despite common side reactions. Over 28 diverse products were made, showing broad functional group tolerance and catalyst flexibility.
8. Why is dioxaza[8]circulene 3a so effective?
Control experiments confirmed the carbazole unit’s key role, but the rigid, fully conjugated, planar circulene framework enhances redox behavior by facilitating charge separation and excited-state stabilization. This structural uniqueness allows 3a to act as a powerful, selective organophotoreductant under mild conditions.
9. Innovation and Impact
This work introduces:
- A green, metal-free electrochemical method to synthesize a novel type of hetero[8]circulenes under mild conditions.
- Oxaza[8]circulenes as a new class of strong organic photoreductants.
10. Future Perspectives:
Our electrochemical platform can be adapted to a wide range of hetero[n]circulene structures with tunable electronic and optical properties. Integrating this approach with asymmetric catalysis and computational design opens new opportunities for advanced materials in optoelectronics and organic synthesis. Continued development of hetero[8]circulene-based photocatalysts promises greener and more efficient routes for sustainable chemical transformations.
11. References:
- Kočovský, P., Vyskočil, Š. & Smrčina, M. Non-symmetrically substituted 1,1′-binaphthyls in enantioselective catalysis. Chem. Rev. 103, 3213–3246 (2003).
- Khalid, M. I., Salem, M. S. H. et al. Electrochemical synthesis of heterodehydro[7]helicenes. Commun. Chem. 5, 166 (2022).
- Salem, M. S. H. et al. Electrochemical synthesis of hetero[7]helicenes containing pyrrole and furan rings via an oxidative heterocoupling and dehydrative cyclization sequence. Adv. Synth. Catal. 365, 373–380 (2023).
- Salem, M. S. H. et al. Data-driven electrochemical one-pot synthesis of double hetero[7]dehydrohelicene. Electrochemistry 91, 112015 (2023).
- Salem, M. S. H., Sabri, A., Khalid, M. I., Sasai, H. & Takizawa, S. Two-step synthesis, structure, and optical features of a double hetero[7]helicene. Molecules 27, 9068 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in