Embryonic-derived macrophages join the party of evil when seats are available
Published in Cancer and Immunology

In this study, we investigated the alterations in Kupffer cells as they infiltrate liver metastatic nodules. Our findings demonstrate that Kupffer cells can be reprogrammed into an inflammatory state due to the inflammatory microenvironment. This transformation is thought to contribute to the immunosuppressive conditions that hinder the treatment of liver metastasis. This discovery may suggest new strategies for cancer therapy that involve the dual blockade of both monocytes and macrophages.
The beginning of the story
My interest in macrophage biology began during my postdoctoral fellowship at the Sloan Kettering Institute, where I was particularly captivated by the heterogeneity of macrophages across various organs and their plasticity in response to different microenvironmental conditions. Upon setting up my own lab and commencing an independent research career at Sun Yat-sen University Cancer Center at the end of 2020, I focused on Kupffer cells, the embryonically derived liver-resident macrophages. Previous studies have suggested that Kupffer cells may play either an anti-tumour1-3 or a pro-tumour role4-6 in liver metastasis. Therefore, my initial objective was to employ more precise mouse genetic models to test which hypothesis is accurate or to reconcile these conflicting findings.
Using multiple lineage tracing models, we discovered that Kupffer cells are unable to infiltrate metastatic nodules, with monocytes serving as the primary source of liver metastasis-associated macrophages. While we were pleased to confirm this observation, previously reported in the landmark paper from Prof. Frederic Geissmann's lab7, it also indicated that our findings may lack novelty. Consequently, we must explore new research directions.
The serendipitous discovery: mechanisms of macrophage replenishment
At that time, we acquired a diphtheria toxin subunit A (DTA)-mediated conditional cell ablation mouse model. Crossing this DTA line with a mouse line expressing cell-specific Cre recombinase results in the genetic depletion of cells expressing Cre recombinase. Given that the fluorescent tracing of monocytes demonstrated significant labelling of liver metastasis-associated macrophages, we employed the monocyte-specific Cre to assess DTA-mediated cell depletion. Surprisingly, we continued to detect a substantial number of macrophages in liver metastasis. This observation suggests that either the DTA mouse line is ineffective or that there are unknown mechanisms that replenish the macrophage pool following monocyte depletion. Meanwhile, another student working on natural killer (NK) cells crossed the DTA mouse with an NK cell-specific Cre mouse and found that NK cells could be nearly 100% depleted, suggesting that the DTA mouse is indeed effective. This raises the question: where do these liver metastasis-associated macrophages originate from?
To further confirm that macrophages can persist even when the external supply of monocytes is cut off, we leveraged the mice lacking Ccr2, the chemokine receptor that functions as an antenna to detect inflammatory signals and mediates the recruitment of monocytes to the sites of inflammation. Consistent with the findings from DTA-mediated cell depletion, the blockade of monocyte recruitment only marginally reduces the number of liver metastasis-associated macrophages.
Inspired by the niche competition model8, we hypothesized that alternative sources of macrophages can compensate for the depletion of monocytes. We can imagine the myeloid niche as a "house". The continuous supply of monocytes to the metastatic tissue leads to their differentiation into macrophages, which subsequently occupy these "houses". When the supply of monocytes is interrupted, we proposed two potential mechanisms to fill these "houses": 1) enhanced proliferation of macrophages within the metastasis, and 2) infiltration of Kupffer cells from the adjacent normal liver. To investigate the first mechanism, we developed a cell-proliferation recording system and demonstrated that a greater number of macrophages undergo proliferation following macrophage depletion. Regarding the second mechanism, we combined Ccr2 knockout with lineage tracing of Kupffer cells, revealing an increased infiltration of Kupffer cell-derived cells into the metastasis in the Ccr2 knockout background (see pictures below).
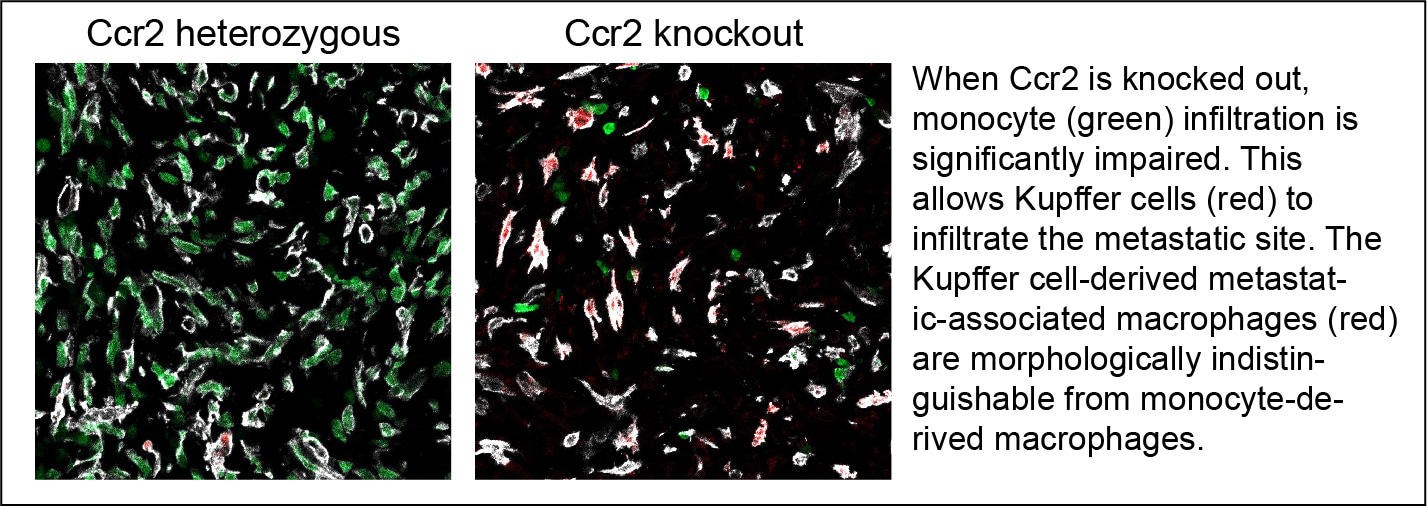
Notably, without our lineage tracing system, which genetically labels Kupffer cells and their descendants with fluorescent proteins, we are unable to distinguish between Kupffer cell-derived metastasis-associated macrophages and monocyte-derived macrophages, particularly as the morphology of Kupffer cells transitions from elongated to flat. In addition to these morphological changes, the metastasis-infiltrating Kupffer cells exhibit similarities to monocyte-derived macrophages in terms of transcriptomic and epigenomic profiles. For instance, Kupffer cells lose their identity markers while acquiring immunosuppressive gene expression in metastatic tissues. We believe this discovery compelling, as it demonstrates that even fully differentiated macrophages can partially erase tissue imprinting and exhibit unexpected plasticity, challenging the previously proposed model9. Furthermore, this plasticity provides an explanation for why Kupffer cells can exert anti-tumour functions in some contexts while mediating pro-tumour effects in others.
Reflections
In addition to the challenges, research is often characterized by unexpected or abnormal observations, which can lead to serendipitous discoveries. Confirming the validity of these unexpected observations and providing explanations for them is often more rewarding than simply verifying anticipated outcomes. However, navigating this journey is fraught with difficulties and frustrations. A crucial quality that has enabled us to complete the project is resilience. As demonstrated in the paper, macrophages exhibit resilience when external supplies are withdrawn; why not you?
Reference
1 Kimura, Y. et al. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc Natl Acad Sci U S A 113, 14097-14102 (2016). https://doi.org:10.1073/pnas.1617903113
2 Thomas, S. K. et al. Kupffer cells prevent pancreatic ductal adenocarcinoma metastasis to the liver in mice. Nat Commun 14, 6330 (2023). https://doi.org:10.1038/s41467-023-41771-z
3 Li, J. et al. The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and antitumor immunity. Nat Immunol 24, 1813-1824 (2023). https://doi.org:10.1038/s41590-023-01634-7
4 Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17, 816-826 (2015). https://doi.org:10.1038/ncb3169
5 Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329-335 (2015). https://doi.org:10.1038/nature15756
6 Wang, G. et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 618, 374-382 (2023). https://doi.org:10.1038/s41586-023-06114-4
7 Deng, Z. et al. The nuclear factor ID3 endows macrophages with a potent anti-tumour activity. Nature 626, 864-873 (2024). https://doi.org:10.1038/s41586-023-06950-4
8 Guilliams, M. & Scott, C. L. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol 17, 451-460 (2017). https://doi.org:10.1038/nri.2017.42
9 Guilliams, M. & Svedberg, F. R. Does tissue imprinting restrict macrophage plasticity? Nat Immunol 22, 118-127 (2021). https://doi.org:10.1038/s41590-020-00849-2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in