Enhancing CO2 electrocatalytic reduction to formate by S-induced water activation
Published in Chemistry
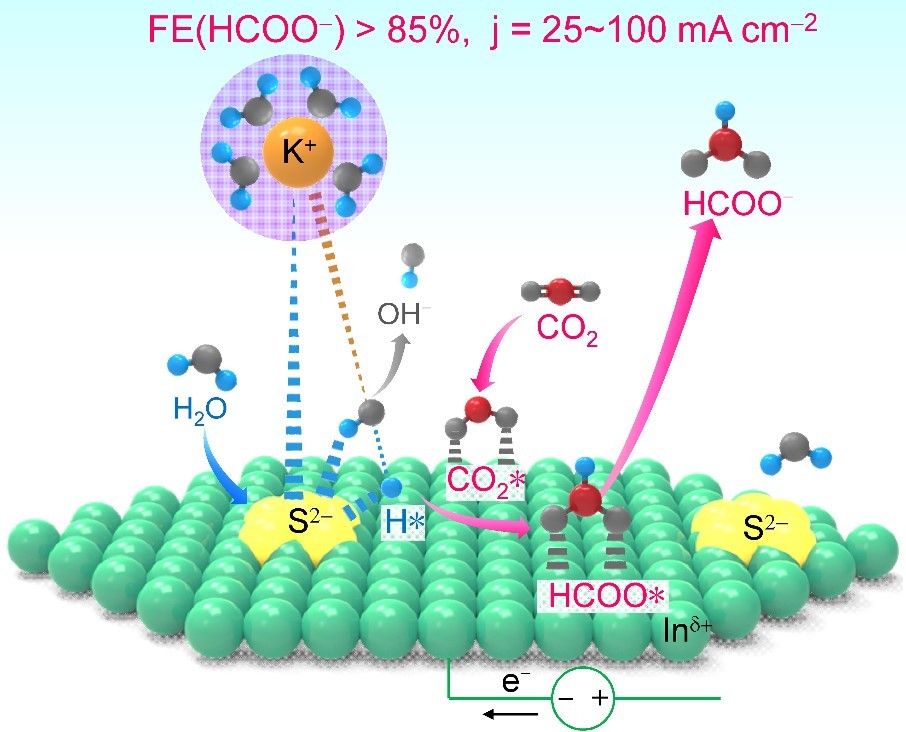
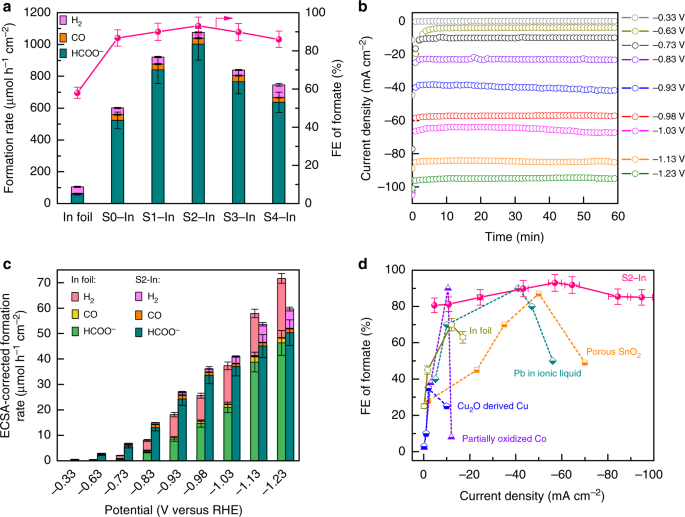
Electrocatalytic reduction of CO2 to fuels and chemicals has attracted much research attention in recent years. Despite significant progress in this area, high faradaic efficiency of a CO2-reduction product can only be achieved at a low current density to suppress the HER reaction, limiting the formation rate of CO2 reduction products. In a recent article published in Nature Communications (https://www.nature.com/articles/s41467-019-08805-x), we have developed a new strategy to enhance CO2 reduction to formate by accelerating H2O activation through sulfur modification of metal catalysts. High faradaic efficiency of formate (>85%) could be kept in a broad range of current density (25-100 mA cm−2) and the formation rate of formate could reach ~1500 μmol h−1 cm−2 with 93% faradaic efficiency, the highest value reported to date.
Catalytic transformation of CO2 to fuels and chemicals provides a promising route to alleviate the rapid consumption of fossil resources and the growing emission of CO2. Electrocatalytic CO2 reduction reaction (CO2RR) has attracted particular attention because of recent progress in generating electricity from renewable energy sources such as solar and wind. Accompanying with CO2RR to formate as an example (Eq. 1), the hydrogen evolution reaction (HER) (Eq. 2) also occurs as a competitive reaction. The current consensus is that the inhibition of HER is essential in obtaining high CO2RR selectivity and formate selectivity. However, the catalysts developed based on this consensus are typically less active in spite of high selectivity. Faradaic efficiency (FE) of formate drops at a high current density (> 60 mA cm-2) because of the significant enhancement in HER, leading to limited formation rate of formate (<1000 μmol h-1 cm-2). Therefore, it would be a significant step forward to develop an effective strategy to accelerate the activity while keeping the high formate selectivity.
CO2 + H2O + 2e− → HCOO− + OH− (1)
2H2O + 2e− → H2 + 2OH− (2)
We have succeeded in developing a powerful sulfur-doped indium catalyst for the electrocatalytic reduction of CO2 to formate with high selectivity at high current density. High FE of formate (>85%) could be kept at a large range of current density (25-100 mA cm−2) for electrocatalytic CO2RR in aqueous alkaline media. The formation rate of formate reaches 1449 μmol h−1 cm−2 with current density of 84 mA cm−2 and FE of 93%. To the best of our knowledge, this is the highest formation rate of formate reported to date for the electrocatalytic CO2RR.
Our studies reveal a unique functioning mechanism of surface sulfur species. The presence of sulfur on indium surfaces enhances CO2RR to formate by accelerating the activation of H2O. We propose that the adsorbed S2− species on indium surfaces can interact with the hydrated metal cations (such as K+ or Cs+) in the double layer, contributing to the dissociation of H2O to form adsorbed H* species, which is responsible for the formation of HCOO* intermediate, the precursor of formate, on indium surfaces. We have demonstrated the universality of the promoting effect of chalcogen species and confirmed that the promoting effect of chalcogen promoters could be extended to other metal catalysts. This work offers a simple and useful strategy for fabricating both active and selective electrocatalysts for CO2 reduction.
A full story of this work can be found in: https://www.nature.com/articles/s41467-019-08805-x
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in