Roles of Cu(I) in H2O activation for electrocatalytic reduction of CO2 to C2+ compoundsCO2
Published in Sustainability
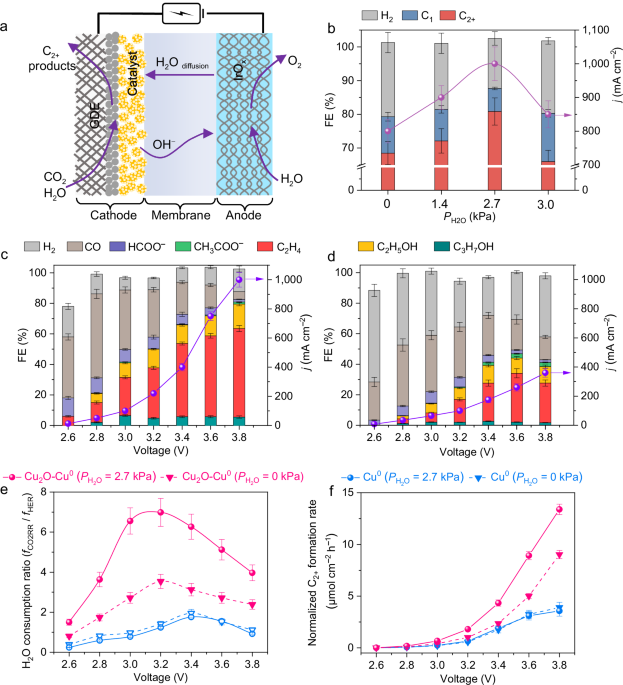
The electrocatalytic CO2 reduction reaction (CO2RR) using renewable electricity to produce multi-carbon (C2+) compounds, in particular ethylene and ethanol, which are widely used in the current chemical and energy industries, is one of the most promising routes to recycle CO2 and reduce carbon emission. Among different electrolysers, the zero-gap membrane electrode assembly (MEA), which has been successfully employed for the polymer electrolyte membrane-based water electrolysis and fuel cells, holds great potential as an energy-efficient and scalable device for CO2 electrolysis. In the MEA electrolyser for CO2RR, the cathode catalyst is directly pressed onto a membrane without a catholyte, but the lack of the catholyte solution makes the management of water, which is the proton source and is essential to CO2RR, in the MEA electrolyser more crucial than in the conventional flow cell. In MEA, the H2O molecules needed for the cathode reaction usually comes from the anolyte by permeation through the membrane and the problem of H2O deficiency may occur at a high current density, worsening the CO2RR performance. However, only very few studies have been devoted to H2O management for MEA-based CO2RR to C2+ compounds. The cathode catalyst is expected to participate in the activation of H2O co-fed with CO2, but the role of the electrocatalyst in H2O management in CO2RR is under explored.
Actually, the lack of catholyte as well as useful insights for the design of cathode catalyst for efficient water management has resulted in limited C2+ formation performances of the MEA-based CO2RR. The current density to attain a C2+ Faradaic efficiency of ≥ 80% is usually lower than 400 mA cm-2, regardless of using a cathode-dry or H2O vapour-feeding MEA system (Supplementary Table 1). It remains challenging to achieve simultaneously a high current density and a high C2+ FE in the full-cell MEA system.
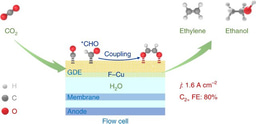
Here, we report our discovery of a suitable electrocatalyst in fulfilling the role of co-feeding H2O in enhancing formation of C2+ compounds during CO2RR in the MEA system. We demonstrate that the co-feeding of H2O has no effect on C2+ formation over a Cu0 nanoparticle catalyst, but a nanocomposite containing abundant Cu2O−Cu0 interfaces shows significantly positive effect of co-feeding H2O on CO2RR to C2+ compounds. Under an optimized H2O pressure, we achieve a current density of 1.0 A cm−2 at 80% FE of C2+ compounds (mainly C2H4 and C2H5OH) in the full-cell MEA electrolyser. The molar carbon-based selectivity and yield of C2+ compounds (C2-3 olefins and oxygenates) reach 83% and 19%, respectively, better than those attained in thermocatalytic systems for CO2 hydrogenation under harsher reaction conditions. Our operando spectroscopic characterizations confirm the presence of Cu+ in the nanocomposite during CO2RR operated at an ampere-level current density in MEA. We propose an in situ re-oxidation mechanism for the formation of Cu+ sites. The catalyst with Cu+ sites promotes the activation of H2O, resulting in enhanced formation of adsorbed CO and CHO intermediates for C−C coupling, and thus showed significant promoting effect of co-feeding H2O for C2+ formation. This work paves the way for constructing an efficient MEA system for CO2RR to C2+ compounds.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in