Exploring HCMV's emerging role as an oncovirus: induction of transformed ovarian epithelial cells by Human Cytomegalovirus (HCMV)
Published in Cancer
Background
Epithelial ovarian cancer (OC), the most common and life-threatening cancer amongst gynecologic malignancies, has a 5-year age-standardized survival rate of 30–40%. Nearly 75% of all OC cases are diagnosed at late stage. Despite the advances in treatment modalities, the overall survival remains low for stage III and stage IV accounting for 40% and 20%, respectively.
Classical OC risk factors including age, family history, epigenetic as well as genetic alterations might generate a tumor-supporting cellular environment, where some oncogenic viruses reside and advance their oncogenic potential. The methyltransferase EZH2 dysregulation was associated with the development, progression, and therapeutic resistance of many tumors, particularly OC. Several studies showed that EZH2 overexpression is associated with high-grade serous ovarian cancer (HGSOC).
HCMV is a herpesvirus that infects between 40% and 95% of the population worldwide; it’s usually asymptomatic. The host immune response retains the virus in a latent stage, however, HCMV can reactivate resulting in sequential lytic/latent viral cycles during lifetime thus contributing to HCMV genomic diversity. HCMV proteins and DNA were detected in glioblastoma tissues, neuroblastoma, breast, ovarian, prostate, and colon cancer underlining the role of HCMV in tumor initiation and/or progression. Establishing a persistent life-long infection, HCMV can modulate signaling pathways linked to mutagenesis, apoptosis, inflammation, angiogenesis, and tumorigenesis. In tumors, HCMV could favor the progression and metastasis of cancer, a paradigm termed oncomodulation. Beyond oncomodulation, an oncogenic role of HCMV has been explored in our research group in which the HCMV will directly transform primary cells especially mammary epithelial cells and astrocytes; such HCMV strains were named high-risk HCMV strains.
Study Objective
In this study, we assessed the transforming potential of the two high-risk clinical strains, HCMV-DB and HCMV-BL, and investigated their molecular and cellular features that appeared in the long-term “CMV-transformed Ovarian cells” (CTO) cultures. Additionally, from HGSOC biopsies that revealed high EZH2 expression, we isolated three clinical HCMV strains that displayed oncogenic and stemness features when cultivated on OECs with enhanced EZH2 expression that could be curtailed by EZH2 inhibitors. Our data support the oncogenic traits of EZH2 thereby permitting the isolation of oncogenic HCMV strains from OC tumors while identifying EZH2 as a potential target for OC treatment, mainly upon HCMV infection.
Key findings
While several research groups have previously given evidence for the oncomodulatory potential of some HCMV strains, hereby, we deliver an important and basic information covering the link between HCMV-induced EZH2 activation and the potential oncogenic role of HCMV in OC (Figure 1).
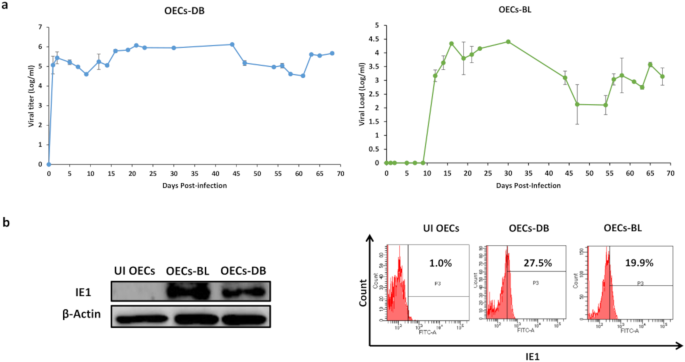
Three major findings were reported. First, primary human ovarian epithelial cells infected with high-risk HCMV strains (DB and BL strains) were transformed with colonies formation in soft agar, displayed stemness phenotype, EMT and tetraploidy/polyploidy with giant cell cycling. HCMV-DB and BL strains were previously identified in our group, and were associated with breast cancer and glioblastoma. In addition, viral genes, transcripts, and proteins of HCMV were detected in the chronically infected CTO cultures. Second, three HCMV strains isolated from HGSOC patient biopsies with high EZH2 expression gave rise to transformed CTO cells in vitro after HCMV infection of the primary human ovarian epithelial cells. Third, a prominent reduction of CTO cells’ growth with the disappearance of tetraploidy/polyploidy in vitro was reported under EZH2 inhibitor therapy.
Closing thoughts
Our data clarify the notion of how HCMV is associated with OC, especially HGSOC. HCMV might not only have oncomodulatory properties in OC. Our results indicated a direct link between HCMV infection and the appearance of OC in patients. Interestingly, the high-risk oncogenic HCMV strains DB and BL transformed primary mature epithelial cells (ovarian and mammary cells) as well as human astrocytes. So far, we isolated 16 high-risk oncogenic clinical HCMV strains directly from tumor biopsies (HGSOCs, n=3; breast cancer, n=2; glioblastoma, n=11). We are currently sequencing these sixteen high-risk HCMV strains expecting to define the viral gene(s) involved in the transformation process. This could indicate that HCMV is an oncovirus. In summary, our findings will pave the way for developing effective therapeutic approaches including EZH2 inhibitors with an improved outcome for patients thus increasing the overall survival rates.
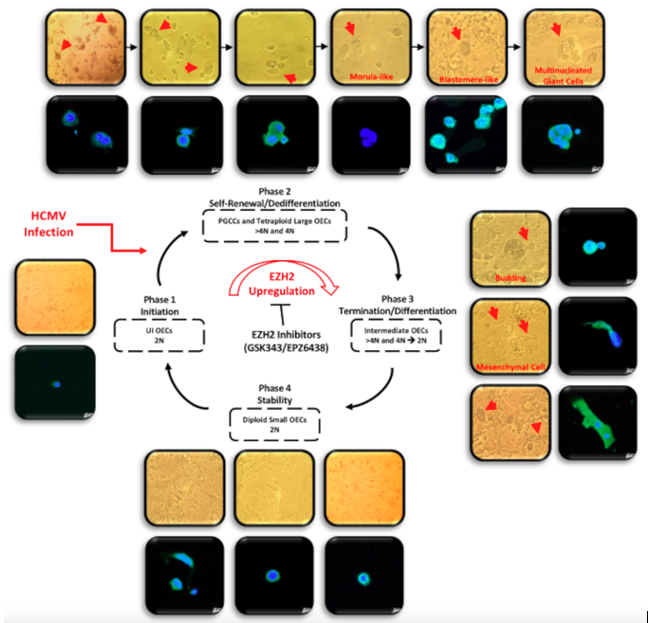
Figure 1. A schematic representing the giant cell cycling following HCMV infection of OECs. Giant cell cycle representing four distinct phases including initiation, self-renewal, termination and stability. Upon HCMV infection, the 2N OECs go into the initiation phase through endoreplication. Polyploid cells (>4 N) and tetraploid cells (4N) generate in the self-renewal/dedifferentiation stage due to HCMV infection and the subsequent EZH2 upregulation. Cell budding takes place from multinucleated or mononucleated giant cells generating intermediate 2–4 N OECs during the termination/differentiation phase. Intermediate OECs gradually reach stability and are converted into diploid small OECs (2N).
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in