FBXW7/GSK3β-mediated proline-rich 11 degradation promotes oxidative DNA damage and inhibits tumor progression in renal cell carcinoma
Published in Cancer
Renal cell carcinoma (RCC) exhibits sensitivity to defects in homologous recombination genes, fueling growing interest in RCC-associated DNA damage [1]. Notably, oxidative DNA damage induced by reactive oxygen species (ROS) is a major contributor to genomic instability [2].
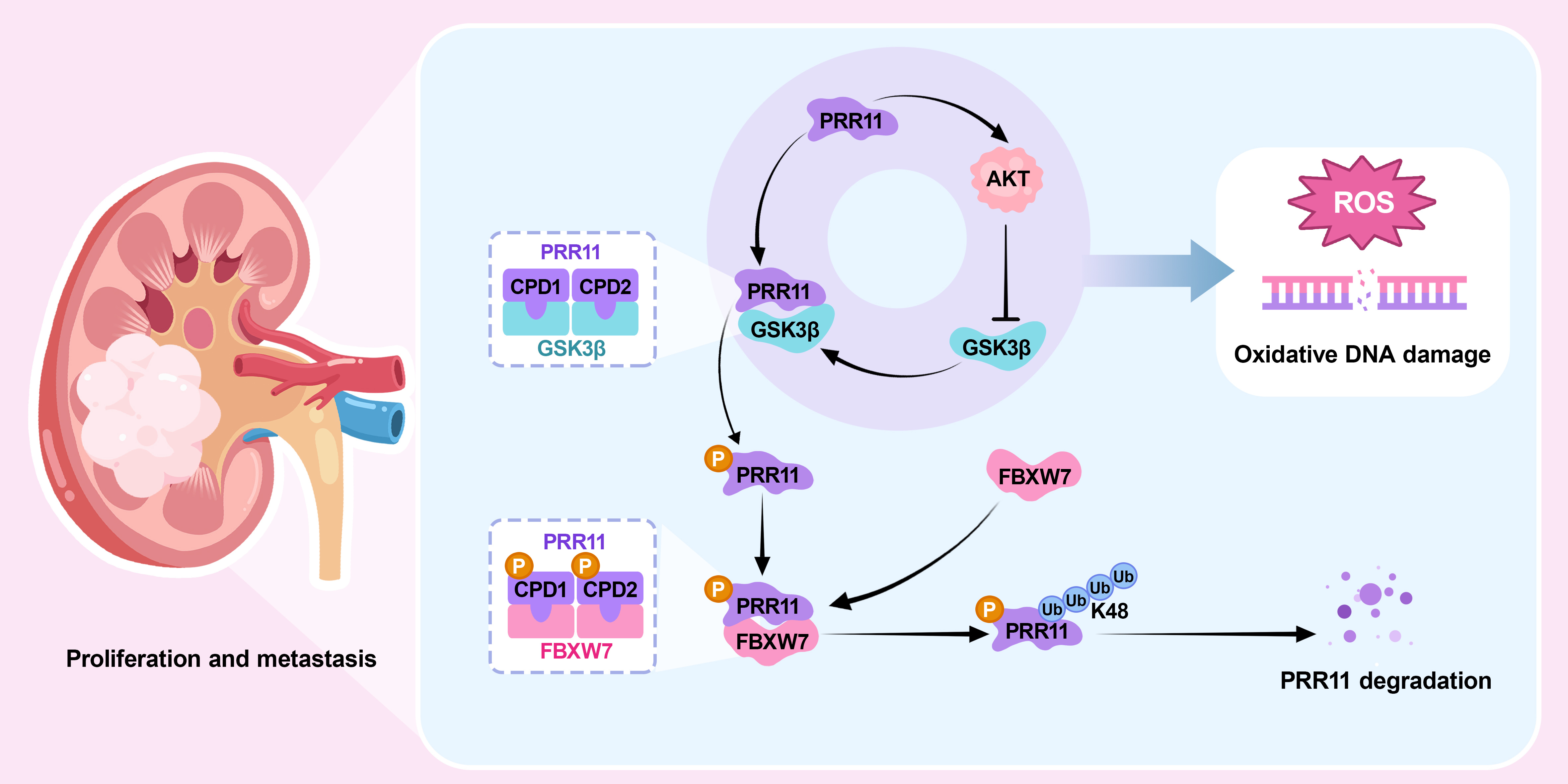
In our previous study, we demonstrated that proline-rich 11 (PRR11) is upregulated in RCC, implicating it in cell cycle regulation and apoptosis [3]. In this follow-up investigation, we explored the procarcinogenic functions of PRR11, with a focus on its role in modulating oxidative DNA damage through the activation of the AKT pathway, thereby promoting RCC proliferation and migration. Mechanistically, GSK3β recognizes and phosphorylates the CDC4 phosphodegron (CPD) consensus motif on PRR11, enabling FBXW7 to bind and facilitate its K48-linked ubiquitination and subsequent proteasomal degradation. Moreover, PRR11 activates AKT signaling, which in turn inhibits GSK3β activity. This inhibition prevents the phosphorylation of the CPD motif on PRR11, obstructing FBXW7-mediated ubiquitination and degradation. This reciprocal interaction between PRR11 and AKT establishes a positive feedback loop that enhances the expression of both proteins and accelerates RCC progression. Our findings elucidate the mechanisms by which FBXW7 and GSK3β regulate PRR11 stability in RCC and reveal how PRR11 influences tumor proliferation and migration through the modulation of oxidative DNA damage. These insights offer promising potential targets for therapeutic intervention in RCC.
References:
[1] Ged Y, Chaim JL, DiNatale RG, et al. DNA damage repair pathway alterations in metastatic clear cell renal cell carcinoma and implications on systemic therapy. J Immunother Cancer. 2020; 8(1): e000230.
[2] Halliwell B, Adhikary A, Dingfelder M, et al. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem Soc Rev. 2021; 50(15): 8355-8360.
[3] Chen S, He Z, Peng T, et al. PRR11 promotes ccRCC tumorigenesis by regulating E2F1 stability. JCI Insight. 2021; 6: e145172.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in