Parkin inhibits proliferation and migration of bladder cancer via ubiquitinating Catalase
Published in Cancer and Cell & Molecular Biology
Bladder cancer (BLCA) is projected to be the fourth most common cancer in men in the United States, with death from BLCA ranking eighth by 2023 [1]. The incidence of BLCA is closely related to risk factors such as smoking and exposure to benzidine. Increasing evidence suggests that the imbalance between oxidants and antioxidants may play a pivotal role in the development of BLCA [2]. Therefore, gaining a comprehensive understanding of the underlying biological mechanisms of BLCA progression and metastasis is crucial in discovering new therapeutic approaches.
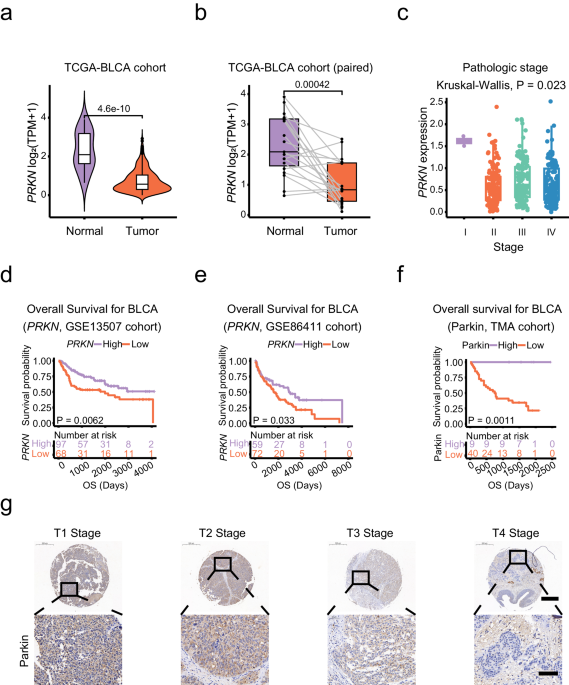
Preliminary sequencing by our group indicated mutations and low expression of Parkin in bladder cancer tissues. Further analysis of public databases and several clinical bladder cancer tissue samples demonstrated a close association between Parkin expression levels and adverse outcomes in bladder cancer patients.
We first verified the suppressive effect of Parkin on the proliferation and migration of bladder cancer cells through cellular experiments. Given Parkin's role as a crucial molecule in the classic process of mitochondrial autophagy, we explored whether the effects of Parkin on the biological behavior of bladder cancer cells involve autophagy mechanisms. Experiments showed that upregulation of Parkin did not significantly activate or enhance the autophagy process. Further research highlighted that changes in the levels of intracellular reactive oxygen species (ROS) were key to Parkin's ability to inhibit the proliferation and migration of bladder cancer cells.
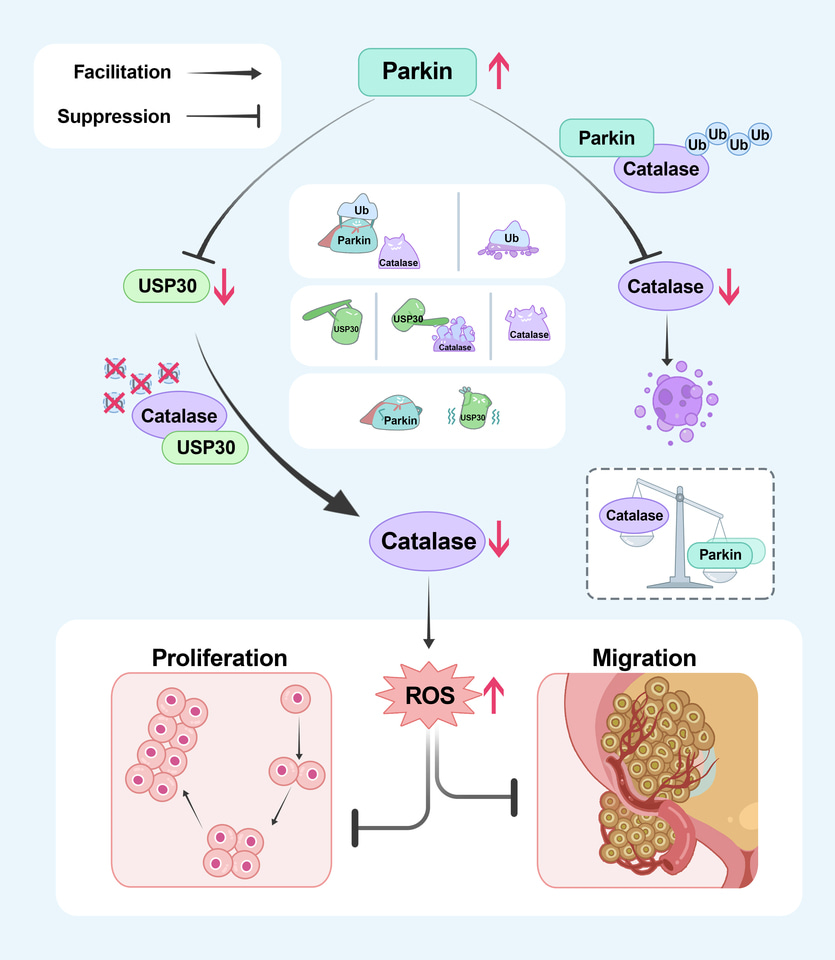
Mechanistic studies found that Parkin interacts with Catalase, promoting its degradation by increasing its ubiquitination levels. Additionally, the research identified USP30, a known substrate of Parkin, as capable of deubiquitinating and stabilizing Catalase. Through these mechanisms, Parkin ultimately reduces Catalase levels, thereby increasing the intracellular ROS content, inducing cell cycle arrest, and inhibiting the proliferation and metastasis of bladder cancer cells.
In summary, this study not only elucidates the role of Parkin as a tumor suppressor in bladder cancer and its mechanism of action but also expands the understanding of Parkin's biological function beyond autophagy. It provides new insights into the role of oxidative stress in the development and progression of bladder cancer. This work suggests that targeting the Parkin-USP30-Catalase pathway could represent a novel molecular therapeutic strategy for bladder cancer.
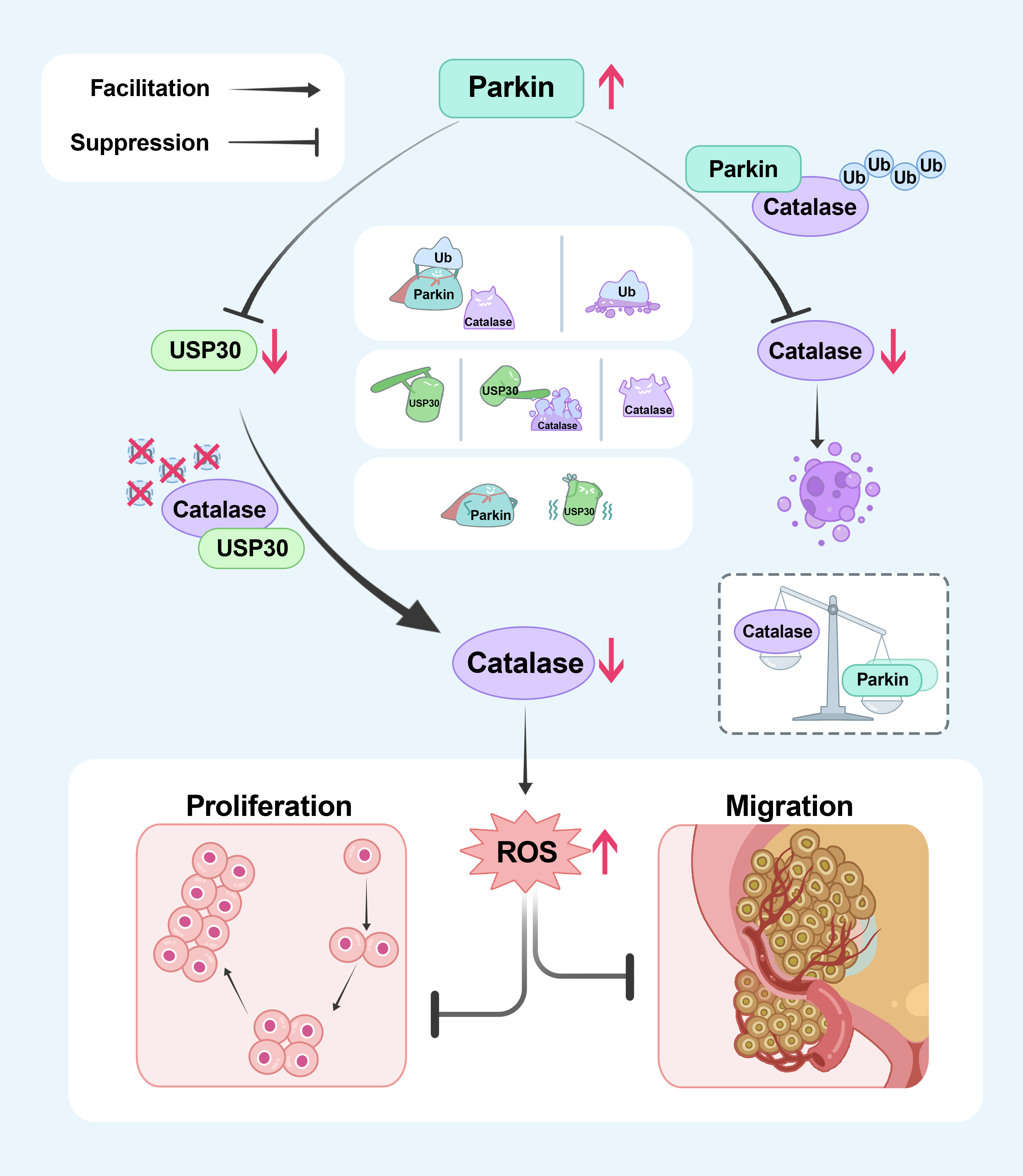
A schematic model of this study. Parkin directly decreases the protein level of Catalase through ubiquitination. Moreover, Parkin degrades the established deubiquitinating enzyme USP30, thereby diminishing its stabilizing effect on catalase through deubiquitination, ultimately resulting in an indirect reduction in the protein level of Catalase.
Dr. Tongzu Liu, Dr. Lingao Ju, and Dr. Xinghuan Wang are the corresponding authors of this paper, with PhD candidate Renjie Zhang, Master student Wenyu Jiang, and Dr. Gang Wang serving as co-first authors.
References
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 73, 17-48, doi:10.3322/caac.21763 (2023).
- Wigner, P., Grebowski, R., Bijak, M., Saluk-Bijak, J. & Szemraj, J. The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. Int J Mol Sci 22, doi:10.3390/ijms22094483 (2021).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in