FGF8 as Director of the Fronto-Temporal Cortical Patterning Orchestra
Published in Neuroscience and Cell & Molecular Biology

Our publication, "A polarised FGF8 source specifies frontotemporal signatures in spatially orientated cell populations of cortical assembloids," conducted in collaboration between the Knoblich and Krenn labs at IMBA (Vienna) and the University of Milan Bicocca, identified a crucial function of Fibroblast Growth Factor 8 (FGF8) in coordinating the human cortex's spatial patterning, specifically in defining the rostro-caudal (front-to-back) axis of cortical regions.
The Prelude: Limitations in Organoid Models
The use of cortical organoids has transformed our ability to study brain development and disease. However, one critical drawback has been the incapacity to recreate the positional information that neural cells display in different cortical regions, within an organoid. This is due to the challenge of in vitro recreating morphogen gradients, like those generated in vivo by so-called secondary organizers, which hinders the investigation of how specific cortical areas develop and how alterations in these processes might lead to neurological disorders.
We sought to address this limitation by engineering a system that would enable the establishment of a lasting morphogen gradient within human cortical organoids. In this study, we focused on FGF8, a major signaling molecule in early telencephalon patterning, which is the brain region that mediates the formation of the cerebral cortex. This was the only factor out of all we tested that could induce the activation of rostral and caudal reporters at different concentrations.
FGF8 as the Conductor: Establishing Axial Patterning in Assembloids
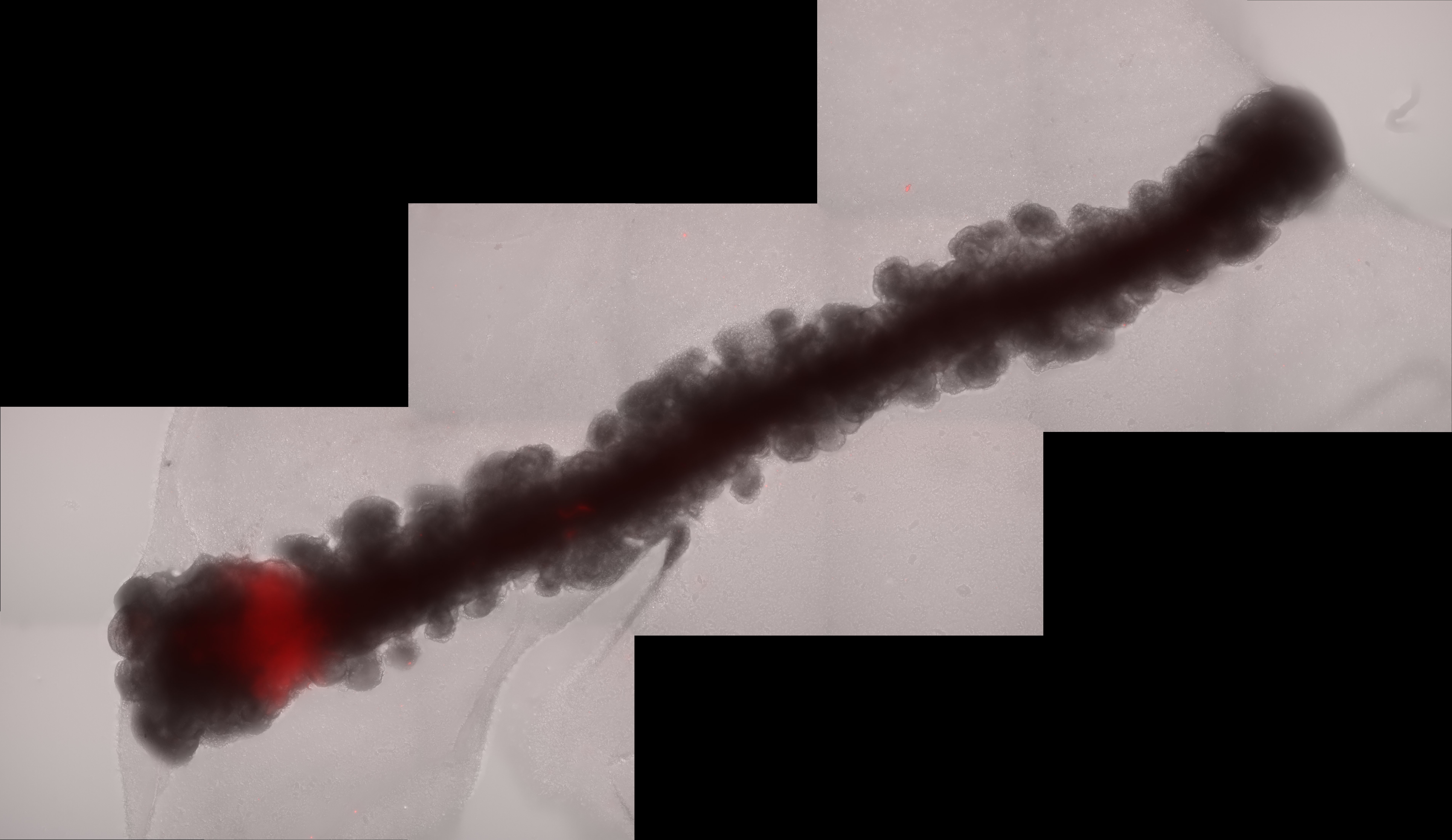
Given the significance of distance in gradient creation in vivo and the absence of gradient-like expression of the frontal reporter in the round assembloid configuration, we postulated that lengthening the organoid could facilitate position-dependent modifications. Therefore, we worked with Tom Wyatt and Benoit Sorre from the Paris University "Matière et Systèmes Complexes" (MSC) laboratory to create a specially designed PDMS mould for the embryoid body aggregation step, resulting in 15mm long linear embryoid bodies. By combining an elongated organoid with an artificial organizer-like structure that constitutively expresses FGF8 (organiser embryoid body, OrEB), we were able to create cortical assembloids with a polarised source (also known as polarised cortical assembloids, or polCA). This design enabled the establishment of a controlled source of FGF8 on one side of the organoid longitudinal axis, effectively mimicking the natural gradient observed in the developing brain.
One of the most remarkable findings from our study was the ability of FGF8 to break symmetry within the organoids, resulting in the formation of distinct cell identities along the proximal-to-distal axis from source. This process, known as symmetry breaking, is a fundamental aspect of developmental biology, prompting initially homogeneous tissues to diversify into different cell types and regions. In our polCAs, the breaking of the symmetry reflected differential expression of genes along the longitudinal axis, with proximal regions showing high levels of expression of genes such as CPNE8 and LMO4 and distal regions expressing NR2F1, LHX2, and FGFR3. This gradient in gene expression, further than validating the model, gives a novel insight on how FGF8 exerts his function in the process of cortical patterning.
The collaboration with the Testa lab from the Human Technopole in Milan and in particular with co-first author Davide Castaldi allowed us to discern the effects of this gradient introduction on assembloids at a single cell level. Single cell RNA-sequencing analysis (scRNA-seq) analysis revealed that our polCAs exhibited a position-dependent transcriptional program that resembled the in vivo rostrocaudal gene expression patterns. This included the establishment of distinct cell populations with gene expression signatures characteristic of frontal and temporal cortical regions. The ability of a single source of FGF8 to induce such precise patterning demonstrates the potency of this morphogen and its critical role in cortical development.
Pathogenic Insights: The FGFR3 Mutation
To further explore the role of FGF8 signaling in cortical development, we introduced a mutation in the FGFR3 gene, which is associated with temporal lobe malformations and intellectual disability in humans. FGFR3 also displayed a low-to-high proximal-to-medial expression in our models. The mutated polCAs failed to establish the normal rostro-caudal patterning, with altered proliferation and disrupted gene expression along the axis. This finding suggests that this FGFR3 mutation impairs the ability of FGF8 to properly direct cortical patterning, providing a potential mechanistic explanation for the cortical abnormalities observed in individuals with these mutations.
A New Model for Studying Cortical Development and Disease
Our study thus not only provides a new model for studying cortical development but also opens the door to exploring the molecular underpinnings of cortical area-specific disorders. By combining our polCA system with gene editing, we can now investigate how specific genetic mutations affect cortical patterning and contribute to neurological disorders. For example, the early patterning defects observed in mutant polCAs could be linked to the transcriptional dysregulation seen across cortical areas in the brains of individuals with autism.
Moreover, our model offers a unique platform for dissecting the crucial aspects of cortical patterning. Manipulating the timing and intensity of morphogens signaling by simply fusing Organizer EBs at different developmental stages or with different percentage of factors-secreting cells, we can begin to unravel how different cortical regions acquire their identities over time and how these processes might go awry in disease.
The Symphony Continues: Future Directions
Although our study identifies FGF8 as the maestro in this cortical patterning symphony, much remains to be worked out. Of particular interest is our observation that, although excitatory neurons still retained a partial temporal area-specific gene signature, other mechanisms must act on top of this and how it intersects with temporal patterning of neuronal differentiation to achieve full cortical area identity. Future work directed toward dissecting these regulatory mechanisms and how the Wnt and RA signaling pathways interact with the FGF8 signal in polCA could provide some answers to these questions.
Follow the Topic
-
Nature Methods

This journal is a forum for the publication of novel methods and significant improvements to tried-and-tested basic research techniques in the life sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Methods development in Cryo-ET and in situ structural determination
Publishing Model: Hybrid
Deadline: Jul 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in