Finding the BI-0115 needle in a compound haystack
Published in Chemistry

Some years ago, based on supporting literature information2-4, we started to investigate the role of the lectin-like oxidized LDL receptor-1 (LOX-1) in cardiovascular diseases and wanted to identify small molecule inhibitors for this challenging target. A small team of scientists in which Heike Neubauer was our biology lead set up all required assays. Most important was an assay measuring the uptake of a fluorescence labelled human oxLDL into a cell line with inducible expression of the human LOX-1 receptor.
The high-throughput screen was orchestrated by Frank Buettner, screening our compound library of more than one million different compounds in the above mentioned cell based fluorescence assay. Early on, it became evident that our hit set was confounded by compounds that merely quenched the dye fluorescence. We knew that we really needed to find the needle in the haystack by weeding out many false positive compounds. For this purpose, we employed beyond a classical biochemical quenching assay also a number of biophysical techniques like surface plasmon resonance (SPR), isothermal titration calorimetry (ITC) and saturation transfer difference (STD)-NMR. Gisela Schnapp and her lab did a great job in producing the recombinant protein, characterizing the hit set and finally identifying the LOX-1 inhibitor BI-0115, our needle in the haystack that turned out to be a gem.
Interestingly, biophysical data already indicated a substoichiometric ratio of ligand to protein. When we were able to solve the structure of a tetrameric LOX-1 complex, which showed that two BI-0115 molecules stabilize a particular arrangement of two LOX-1 receptor dimers, we had our “Heureka!” moment that all drug discovery scientists are striving for. We identified an as of yet unknown mode-of-action showing that BI-0115 acts as a molecular glue and pins two receptor dimers together incapacitating them from binding their physiological ligand.
To exclude the possibility that this finding was an artefact of crystallization, in the following, it was important to show that this 4:2 complex also truly exists in solution. Here, we asked Francois Debaene and his colleagues from NovAlix if they could help us by their remarkable non-denaturing Electrospray-Ionization Mass Spectrometry (ESI-MS) technique. Indeed, they could!
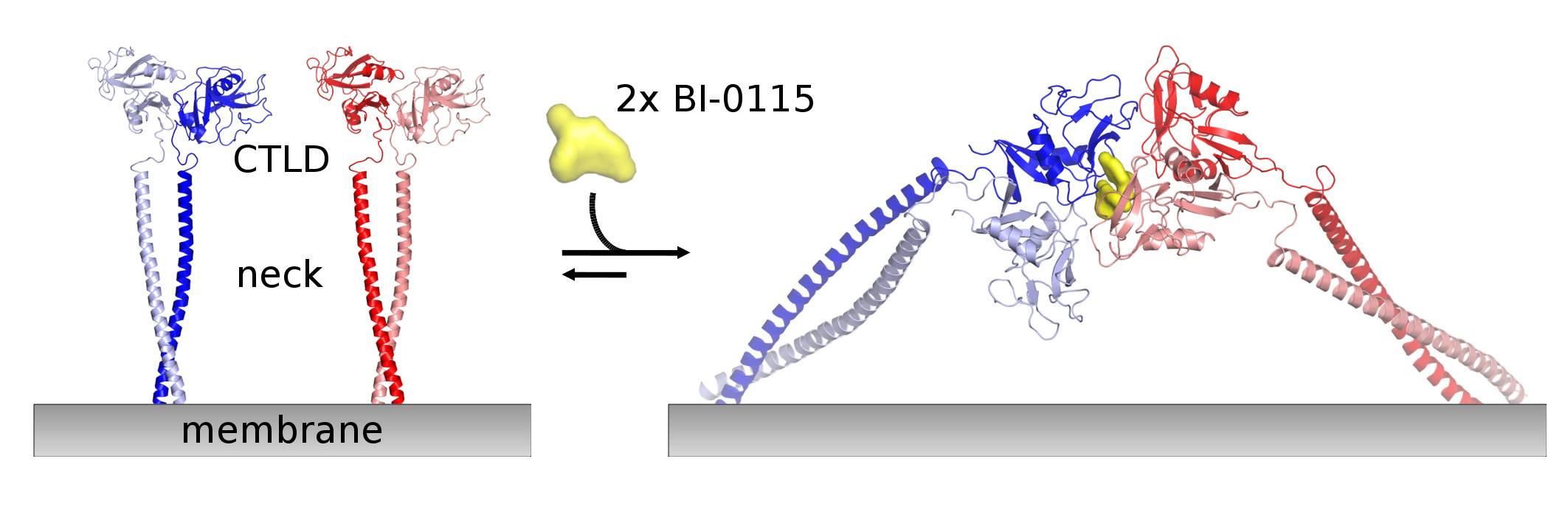
Molecular glues have become very popular in recent years with the most prominent example of PROTACs (proteolysis targeting chimeras). We show in our work that inhibition by stabilization of multimeric states might be an attractive way to target a family of receptors believed to be very difficult to drug.
If we got you interested in this new mechanism of LOX-1 inhibition, please read our paper for more details. Furthermore, we share the BI-0115 compound with the scientific community via opnMe.com, the Boehringer Ingelheim Open Innovation portal.
Working on a project in a pharmaceutical company always involves a team of many people from different disciplines. We would like to say thanks to all that have contributed.
References:
- Chiffoleau, E. C-Type Lectin-Like Receptors As Emerging Orchestrators of Sterile Inflammation Represent Potential Therapeutic Targets. Front Immunol 9, 227 (2018).
- Suzuki, T. et al. Diagnostic implications of circulating oxidized low density lipoprotein levels as a biochemical risk marker of coronary artery disease. Clinical Biochemistry 35, 347-353 (2002).
- Sawamura, T. et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73-77 (1997).
- Dunn, S. et al. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J 409, 349-55 (2008).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in