From Sawmills to Robotic Brain Stimulation: A Personal Quest to Rewire Addiction
Published in Neuroscience, Biomedical Research, and General & Internal Medicine
Why this research matters
This story begins on a rainy night on Vancouver Island in the mid-1990s. I'd just been laid off from my sawmill job and was heading to Alberta to work on oil rigs. My older brother was contemplating rehab for his crack cocaine addiction. Like several of my friends during the cocaine epidemic, he'd lost everything—house, job, relationships.
I asked what seemed simple: "Why can't you just quit?"
His answer stuck with me for decades. He'd tried repeatedly, but any trigger—a thought, a person he'd used with, even a smell—would spark an unstoppable urge that wouldn't quit until he got the drug, no matter the cost. He went to rehab and stayed clean for a few years. First relapse occurred when he ran into an old friend. Then a few years later a doctor prescribed OxyContin for a back injury. He lost his family, cycled through homelessness, and for 10 years we had no relationship. Last summer, we reconnected. He'd survived 30 overdoses during the opioid epidemic. He's on methadone now, trying to rebuild. Thirty years into his struggle, we sat outside on a sunny day at our mom's place, and he described his opioid addiction the same way, but worse: uncontrollable motivations triggered by cues, driving him to relapse over and over—a loss of control.
That question—"Why can't you just quit?"—became the question I couldn't let go. Today, I run a lab at Rutgers University where we investigate how drugs of abuse hijack brain reward circuits to drive the addiction cycle, and develop new brain-based treatments to restore normal function. Our recent study in Translational Psychiatry shows we can actually normalize reward responses in people who use opioids using robot-assisted brain stimulation, and we hope this will lead to a future treatment for substance use disorders.
What we did and what we found
In graduate school, I learned the neuroscience behind my brother's response to my question. Your brain learns what's valuable through dopamine signals called reward prediction errors (RPEs). These signals are sent to a part of the brain called the midcingulate cortex (MCC), and can be measured using a brain signal called the reward positivity. Importantly, these signals teach the MCC and other brain regions which cues and behaviors lead to rewards and motivate you to select and pursue those actions towards goals.
Unfortunately drugs of abuse, like cocaine and opioids, hijack this learning process. While normal rewards only trigger dopamine when unexpected, most drugs of abuse keep increasing dopamine even when fully expected. This creates exaggerated RPE signals that never stop, continuously reinforcing drug cues and enhancing the motivation to select drug-seeking behaviors, while making the brain numb to natural rewards like relationships, work, or health. Our previous studies have shown that people with a substance use disorder, including opioid use, showed a flatlined response to monetary rewards. This means their brains weren't responding to normal incentives anymore. Such abnormal MCC functioning would underlie decreased valuation and effortful pursuit of a wide range of pro-social goals, like abstinence if you are trying to quit the drug, and a marked narrowing of life goals to obtaining and using drugs of abuse.
After years of observing this blunted response, I kept thinking: can we fix it?
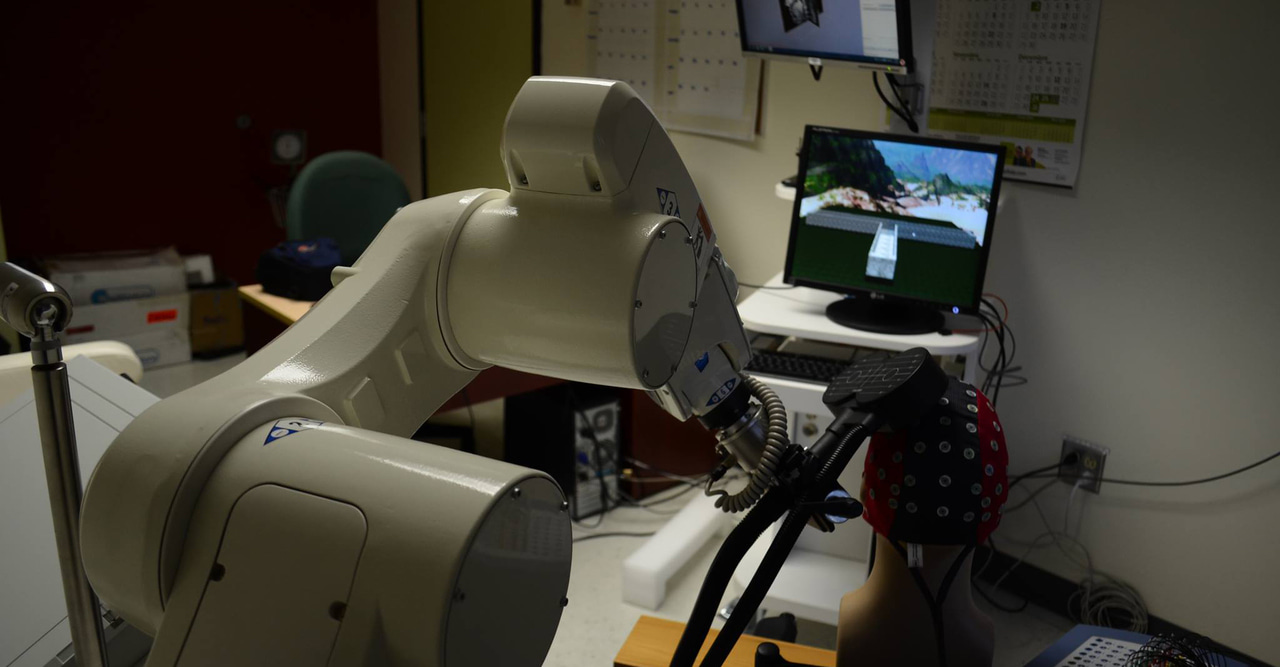
That's where transcranial magnetic stimulation (TMS) comes in. TMS uses magnetic pulses to temporarily enhance brain activity, particularly dopamine release and neural activity in reward related regions, such as the cingulate cortex and basal ganglia. It's been successful treating depression. But conventional TMS has a problem: move your head slightly, and you've changed which circuits you're affecting. So we turned to robot-assisted TMS. A robotic arm holds the magnetic coil outside the skull, tracking head position in real-time with sub-millimeter precision. If someone moves, the robot adjusts instantly and keeps the stimulation precisely on the circuit you want to target.
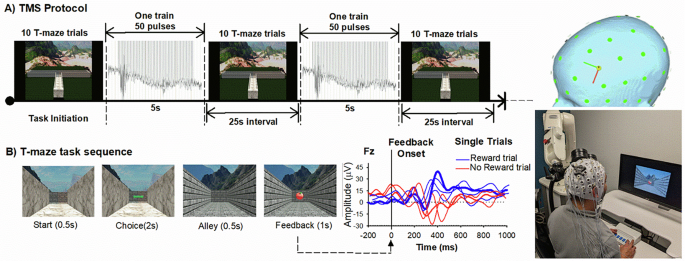
When Kathryn Biernacki PhD joined my lab as a post-doctoral researcher, she brought with her a strong background in opioid use disorder and its impact on decision-making, as well as a drive to improve the lives of people living with this disorder. She was a perfect fit for this project. Despite COVID-related shutdowns and restrictions, Kathryn managed to recruit 80 participants with opioid use histories, and half healthy controls. We stimulated each participants’ prefrontal cortex with 2000 TMS pulses over 20 minutes while participants completed a reward task and we monitored their brain activity. To make sure the effects of TMS weren’t a fluke, we gave half the participants “active” TMS and half “placebo” TMS.
The results were remarkable. People who use opioids receiving placebo TMS showed the same blunted reward response we had seen in our other studies. But those who received active TMS showed a completely normalized response—their brain's reward signal looked indistinguishable from healthy controls. The robot-assisted stimulation had, at least temporarily, normalized their brain's reward system. What made this compelling was its specificity: it worked on the exact reward signal we targeted, and it worked whether participants were currently using or had been clean for months or years. To understand whether the flatlined reward signal also reflected actual structural damage to the MCC, we collaborated with Drs. Rita Goldstein and Alia-Klein Nelly to analyze brain imaging data from a separate cohort of individuals with opioid use disorder. The results were striking: people with opioid use disorder showed significant cortical thinning in the MCC compared to healthy controls—the very brain region generating the flatlined reward signal. This confirmed we weren't just seeing functional impairment; the MCC itself was structurally affected.

Figure: The moment we saw it. With first author Kathryn Biernacki (right) and co-author Malte Güth (left), seeing our results laid out for the first time. Active TMS had normalized the reward positivity in opioid users—the brain's reward response looked indistinguishable from healthy controls. After years of research showing blunted reward signals, we'd found a way to restore them.
What this means and where we go next
TMS isn't a magic cure. Addiction is complex, involving psychological, social, and biological factors. But we've shown we can measure and modify specific brain circuits involved in the maladaptive motivational and decision making patterns that sustain the addiction cycle. Think of it this way: current treatments are like building a house on a cracked foundation. Medications like methadone and buprenorphine are crucial—they stabilize people, reduce harm, reduce cravings, and prevent withdrawal. But if the brain's fundamental goal-directed system remain broken, maintaining recovery is an uphill battle. What if we could repair that foundation?
We're not just suppressing symptoms. We're trying to help the brain work like it used to, to restore the capacity for pursuing a full range of life goals. We are now trying to get funding to test whether repeated TMS sessions produce lasting changes. Can we help people regain the ability to value and pursue meaningful goals? Can we make it easier to stay engaged in treatment and maintain treatment goals?
I dedicate this work to my brother, who sparked the question that became the driving force behind this research. He survived both the cocaine epidemic of the 1990s and the opioid epidemic that followed. It took 30 years for me to get here, and 30 years for him to come clean for over 2 years. I hope this work will help accelerate treatment progress for opioid use disorder and other substance use disorders, so that others don't have to wait three decades for a brain-based treatment that works.
The opioid crisis continues to devastate communities worldwide. From sawmills to robotic brain stimulation, from not knowing what graduate school was to running my own lab—it's been quite a journey. But the question remains: why can't people just quit? Now, at least, we're beginning to understand the answer. And more importantly, we're starting to develop tools that might actually help the recovery process.
Biernacki, K., Goldstein, R.Z., Güth, M.R. Nelly, A., Cole, S., Suchismita, R., Baker, T.E. Blunted anterior midcingulate response to reward in opioid users is normalized by prefrontal transcranial magnetic stimulation. Transl Psychiatry 15, 340 (2025). https://doi.org/10.1038/s41398-025-03569-z
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in