Glutathione and Iron Interplay in Alzheimer's: A Clue for Intervention?
Published in Neuroscience

Alzheimer’s disease (AD) is renowned as one of the most maleficent neurodegenerative disorders and is a major cause of dementia that slowly destroys the daily life of afflicted individuals. It is often detected after the damage is irreversible. Presently, there is no definitive cure. With an increasing prevalence globally, the causal mechanisms for AD are attributed to unpredictable microenvironment changes in the brain. Ever since Alois Alzheimer described the disease in 1906, its incidence has only progressively increased with more than 55 million people afflicted with dementia currently (Dementia Fact Sheet, WHO, March 2023). AD mostly affects those over 65 years of age, however, people in their late forties can develop the prodromal stage of AD- mild cognitive impairment (MCI).
Extensive research is ongoing on a war footing to find the causative factor for AD progression, and eventually a cure. Two theories are most prominent- the amyloid beta (Aβ) and the tau phosphorylation hypothesis. Early detection of AD and lifestyle changes may be helpful in alleviating some of the early symptoms of the disease. Early susceptibility biomarkers, therefore, become extremely important.
Situated at some distance from the energetic city of Gurugram in the National Capital Region, India, is our research institute- the National Brain Research Centre (NBRC). Modest, yet productive in its research, NBRC is the foremost research institute in the country dedicated solely to studying the brain and investigating its many mysteries. The Neuroimaging and Neurospectroscopy Laboratory (NINS Lab), spearheaded by Prof. Pravat K. Mandal, has its specific purpose of designing precise tools for the early detection of AD. The overarching goal in our lab has been to investigate the role of oxidative stress (OS) in the pathogenesis of AD.
OS is commonly defined as an imbalance of antioxidants and prooxidants that increases the oxidative burden on the physiological system. Glutathione (GSH) is a tripeptide with the constituent amino acids glutamate, cysteine, and glycine. GSH is able to sequester reactive radicals and eliminate them to reduce OS in living systems. GSH is also a major antioxidant in the brain. On the other hand, iron is a prooxidant and can increase OS in the brain by participating in Fenton’s reaction and contributing to the formation of reactive oxygen species (ROS). The sea horse-shaped hippocampus, the learning and memory center of the brain is highly susceptible to OS and is markedly affected in AD patients. A study from our NINS laboratory found significant depletion of GSH and enhanced iron levels in the hippocampus of AD patients [1]. Another study from UC Irvine recently validated that indeed GSH depletion precedes plaque deposition in transgenic AD mice [2]. This provides evidence that OS might be the starting point, leading to further downstream processes like Aβ plaque deposition and tau hyperphosphorylation.
GSH is distributed non-uniformly across different brain regions in both healthy young and old persons [3]. This is probably due to the differing levels of susceptibility of different brain regions to OS. However, the concentration of GSH in these brain areas is maintained in healthy people. In fact, we conducted a study to measure and find a correlation between brain and blood GSH and iron levels. We had two major findings. Firstly, plasma GSH, and serum iron levels were similar across four age groups (18y to 72y) in healthy population. Interestingly, the same trend was observed for brain GSH and iron levels [4]. Analysis of relevant literature along with the results from this study helped us to establish the hypothesis that a low GSH/iron in the blood plasma and serum respectively may serve as potential biomarkers for the risk of developing AD. Validation with longitudinal studies with a larger population size would further help to develop early screening tools for AD based on the same idea and is under process in our lab.
Even though it has been over a century since this devastating disease was discovered and despite massive research efforts over many long years, the specific cause of AD remains an enigma. The most popular hypothesis has been attributed to is the deposition of Aβ plaques in the brain. Drugs developed based on this hypothesis, however, have not met with much success in recent times. Some drug trials based on the Aβ cascade have documented adverse side effects like brain hemorrhages. A novel approach to AD drug development is therefore critical.
Our laboratory has proposed a combinational drug therapy involving combined GSH supplementation and iron chelation for upcoming clinical trials for AD patients. Previously, oral GSH administration has been observed to improve cognitive decline and behavioral depression in mice. Our plan is to conduct future human trials to test whether GSH supplements along with controlled iron chelation have similar affects in human counterparts.
We aim to provide a comprehensive research initiative: GLUTASCAN for AD patients as well as to detect MCI in early stages based on our previous study [4]. There are chances of improvement by enriching brain GSH levels in MCI patients. Longitudinal detection of GSH level in the left hippocampus by the state-of-the-art non-invasive MEGA-PRESS sequence and quantification of GSH level using KALPANA package [5] are important components of this initiative.
In conclusion, the research outcomes of this novel initiative are likely to enhance the quality of life for individuals suffering from AD.
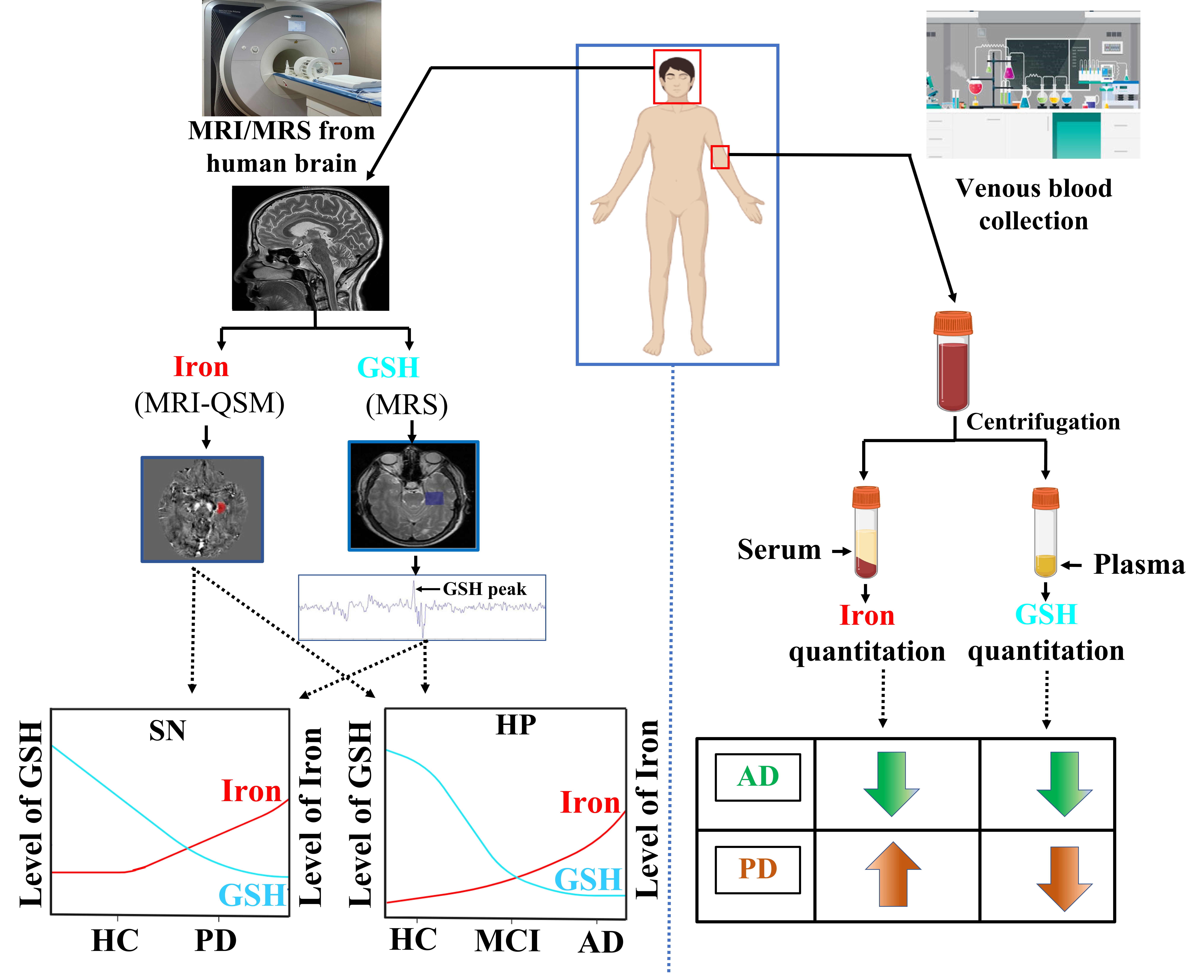
A schematic representation of the workflow of GSH and iron quantitation from brain and blood. Based on literature review of AD and PD patients' studies, GSH and iron levels in brain and plasma and serum is represented.

Follow the Topic
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in