Go green or go home! It’s time to consider the solvents we use in printed electronics
Published in Electrical & Electronic Engineering

Well, we have! And, as a result we developed a simple tool that helps you find suitable benign solvents for your projects.
The true era of printed electronics is upon us, with almost endless possibilities. It is no longer farfetched to talk about cheap and easily fabricated electronic and photonic devices, using standard and accessible solution-based printing techniques. A steppingstone for this envisionment is the emergence of organic (carbon-based) semiconducting materials. Their beauty is that we can specifically tailor their electronic and optical properties to suit various applications but also, importantly, their size and solubility, so that we can use energy- and cost-efficient solution-based fabrication methods.
However, when working in a research lab in the field of printed electronics, we tend to focus on these active materials and somewhat neglect the impact of the solvents we employ (although they often make up most of the material used). We normally use the solvent that we “know” works well, although some of the solvents can be really nasty and dangerous from either health, safety and/or environmental perspectives. That is generally not such a big problem since we deal with so small quantities that they are somewhat easy to manage from all perspectives. However, as the research goal is to scale-up and commercial fabrication, the choice of solvent really becomes important. For instance, you do not want the workers to be exposed to harmful solvent vapors while claiming the sustainability advantages of the fantastic applications made with organic semiconductors.
Although we have been aware of the solvent challenge, we never really did anything about it, until we kept getting asked “what solvents do you use?” from industrial partners. Their concerns intrigued our curiosity to investigate possible functional and environmentally benign solvents, fittingly referred to as “green” solvents, and thereby facilitate the transition to an up-scaled production. When doing so we realized that it was more cumbersome than we had thought, and that is why we decided to develop an easy-to-use, accessible and intuitive tool which can help the entire organic electronics community to go green(er)!
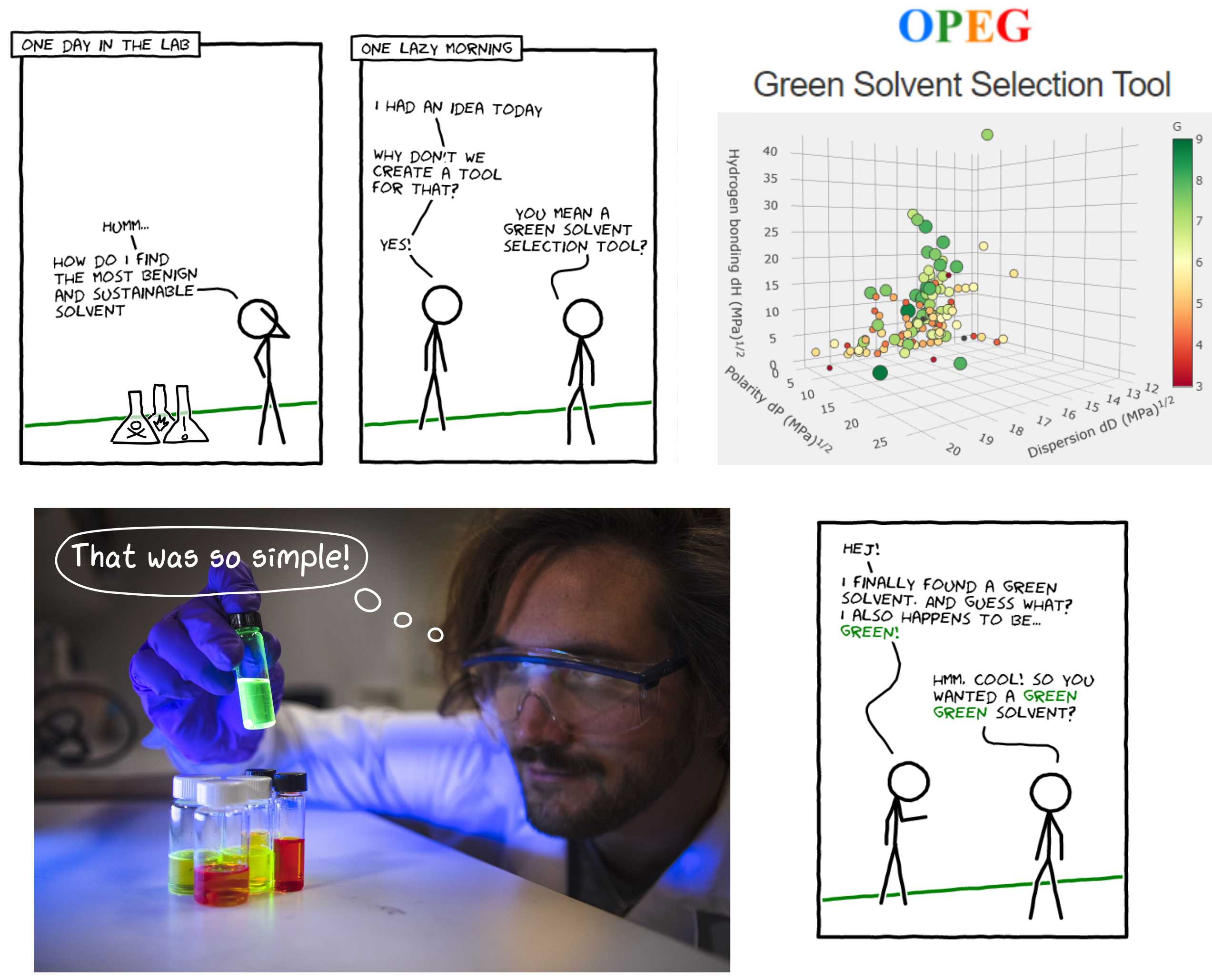
So, what is a green solvent? Well, there is no trivial answer to that question, but it is reasonable to think that it should be safe to use and have a small environmental impact. Luckily, we found that there are already quite a few solvent sustainability guides out there—some that are regularly updated. Even better is that they tend to agree, which is reassuring. After scanning through the various guides, we selected the one from GlaxoSmithKline (GSK),1,2 as their approach to rank solvents is transparent and well described. In this guide, the solvents are graded according to four categories: health, safety, environmental impact, and waste disposal. By combining the score on each of these categories they get an overall score, denoted G, which indicates how green the solvent is. The higher the score, the greener solvent. Might sound simple, but there is in fact a lot of solvent properties and GHS (Globally Harmonized System of Classification and Labelling of Chemicals) data going into each category.
So, we get an indicator of “greenness” from the GSK guide. But sadly, it is not as simple as just picking the greenest solvent… It also needs to be functional! That is when it struck us: we can make use of Hansen’s solubility theory—which is kind of a fancy like-dissolves-like concept—to see which solvents behave similarly and thereby easily change from a problematic solvent that we know works to a more benign solvent, without losing functionality.
Then by combining these two pieces into a tool, adding relevant solvent property data and other useful stuff (in part thanks to excellent input from the reviewers), we got the OPEG Green Solvent Selection Tool. We tried our best to make the tool simple and self-explanatory, and honestly, we are really happy with how it turned out! We will not go into details here (you can find it in the published paper and under “How it works?” in the tool), but basically you only need one of two things, either: the Hansen solubility parameters of your solute (which we rarely have when working with new materials) or knowing at least one solvent that works for your system. From that, the tool will guide you towards a greener solvent. It is that simple!
After designing the tool, we still needed to test that it worked, so we performed a case-study with one of the most complex inks we could think of: a multicomponent high-performance light-emitting electrochemical cell (LEC) ink consisting of a (1) blend-host (small molecule and polymer), (2) small molecule Ir-based guest, and (3) ionic liquid. When fabricating LECs from such an ink we previously used chlorobenzene (G = 5.4), a problematic solvent since it is harmful to both skin and eyes, and when inhaled, as well as toxic to aquatic life and flammable. Using our Green Solvent Selection Tool, we could directly identify a set of greener alternative solvents that were likely to work (since they have similar Hansen solubility parameters), i.e., ethoxybenzene (G = 7.2) and anisole (G = 7.4). We tried them out by fabricating both spin-coated and bar-coated devices—a much more scalable fabrication method—from inks based on the greener alternatives, and indeed the method worked. The devices fabricated using both fresh and stored inks from the different solvents behaved essentially identical. Success!
So, why wait to go green until it is requested? Isn't it smarter to skip the intermediate stage of development? It is not difficult, and it makes your work environment safer and your research more sustainable. So go green or go home! ;)
1 Alder, C. M. et al. Updating and further expanding GSK's solvent sustainability guide. Green Chemistry 18, 3879-3890, doi:10.1039/c6gc00611f (2016).
2 Henderson, R. K. et al. Expanding GSK's solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry. Green Chemistry 13, 854, doi:10.1039/c0gc00918k (2011).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in