Harnessing the interactions between light and nanoparticles: expanding the therapeutics options for glioblastoma treatment
Published in Cancer
Glioblastoma multiforme (GBM) is one of the most malignant forms of central nervous system (CNS) tumors. Despite the progress made in developing new treatment approaches, it continues to remain predominantly incurable1. In addition to the heterogeneous and immunosuppressive nature2,3, a significant challenge in traditional therapies lies in the difficulty of achieving sufficient drug concentrations in the tumor due to the protective blood-brain barrier (BBB). Formed by a tight-junction (TJ) protein complex and adherens junctions between brain microvascular endothelial cells and regulated by surrounding stromal cells like pericytes and astrocytes, the BBB maintains normal metabolic activity and neuronal function in the brain. On the other hand, the BBB also prevents more than 98% of small-molecule drugs and all macromolecular therapeutics from entering the brain4. Though GBM can disrupt the BBB in its hypoxic and angiogenic core, this local disruption is nonuniform or insufficient to enable substantial drug penetration. Additionally, evidence suggests that GBM's tumor cells infiltrate neighboring tissues without disrupting the BBB5. Therefore, drugs with poor BBB permeability do not provide therapeutically effective drug exposures to this fraction of tumor cells, leading to inevitable recurrence.
To date, a variety of strategies have been developed to overcome BBTB for therapeutic delivery. Nevertheless, GBM is still considered to be incurable using currently approved treatments and many promising results fail at the clinical trial stage. Thus we believe that it is critical to develop and validate treatment strategies with clinically relevant GBM models to bridge the gap between preclinical efficacy and successful clinical translation.
Our Approach
To address this issue, we collaborated with Dr. Robert Bachoo at the University of Texas Medical Center, who developed two primary conditional mouse cell lines (namely PS5A1 and 73C) that carry mutations seen in both adult and pediatric high-grade gliomas (namely, (1) BrafV600E, INK4ab/Arf-/-, PTEN-/-, for PS5A, and (2) BrafV600E, P53-/-, PTEN-/-, for 73C). We further established two genetically engineered mouse models (GEMMs) using these cell lines, and characterized their properties such as BBTB integrity and tumor growth patterns.
To modulate the BBTB permeability, we first synthesized and characterized nanoparticles (AuNP-BV11) that target one of TJ components, JAM-A. These nanoparticles were i.v. injected into a tumor-bearing mouse, followed by the delivery of a transcranial 532 nm picosecond laser pulse to the tumor region to stimulate the AuNPs for BBTB modulation (optoBBTB, Fig. 1). We then systemically delivered fluorescent dyes or therapeutics to investigate the BBTB permeability and brain uptake. For GBM treatment, we optimized the optoBBTB parameters to achieve the maximum opening efficiency in each GEMM, and evaluated the brain delivery and efficacy of an oncology drug paclitaxel (Taxol). Taxol is selected since it is among the most widely used oncology drug because of its proven efficacy in multiple cancer subtypes. However, it was abandoned for GBM treatment due to its failure in early-phase clinical trials presumably because of the poor brain penetration.
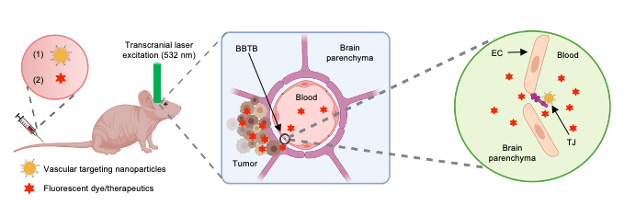
Fig. 1. Schematic illustration of optoBBTB. EC: endothelial cell. TJ: tight junction. The illustration figure was created with Biorender.com.
Key findings and take-home message
Our study demonstrated that the PS5A1 GEMM maintains an intact BBTB throughout the disease progression, coupled with an infiltrative growth pattern (vessel co-option), while the 73C GEMM shows an angiogenic tumor core and intratumoral heterogeneity in BBTB function and TJ composition. Collectively, these two GEMMs recapitulate the tumor-stromal phenotype observed in human GBM patients. We further demonstrated that three cycles of optoBBTB followed by Taxol delivery suppress tumor growth (6 and 2.4- fold) by reducing tumor cell proliferation and increasing cell death, leading to significantly improved median survival (50% and 33% increase) in PS5A1 and 73C GEMMs, respectively. These results demonstrate that optoBBTB is effective for drug delivery and GBM treatment in two clinically relevant GEMMs.
Our efforts towards GEMMs development and characterization hold extensive implications, since it is critical to develop strategies that can overcome the BBTB both in the angiogenic tumor core and infiltrative tumor margins in order to achieve a significant improvement in drug delivery and patient survival. The combination of optoBBTB and clinically relevant GEMMs offer valuable insights for future GBM treatments, functioning as a drug development and screening platform for testing a class of potent anticancer drugs for superficial tumors.
Future directions
Future research can be focused on targeting tumors in deep brain regions by exploiting near-infrared laser and near-infrared light-absorbing nanoparticles to improve the light penetration depth in the tissue. Also, it will be of interest to evaluate the feasibility of using optical fiber in the tumor surgical cavity for light delivery into deeper brain regions.
The team behind the study
The team behind this study spanned researchers in different stages of their careers with complementary professional backgrounds. The lead of the study, Dr. Qi Cai, was a research associate at the University of Texas at Dallas with training in chemistry, bio-nano interactions, and CNS drug delivery, and is now starting her lab at Lousiana State University. The Principal Investigator, Dr. Zhenpeng Qin, is an Associate Professor of Mechanical Engineering and Bioengineering at the University of Texas at Dallas, and of Biomedical Engineering at UT Southwestern Medical Center. Dr. Qin has expertise in studying fundamental biotransport for the brain and developing nanotechnologies to put medicines into the brain. The team included expertise from a physician-scientist Dr. Robert Bachoo, Associate Professor of Neurology at UT Southwestern Medical Center, and holds joint appointments in the Department of Internal Medicine and the Simmons Comprehensive Cancer Center. Dr. Bachoo’s research focuses on understanding the fundamental mechanisms that drive malignant brain tumors. The team also included complementary expertise in engineering and nanotechnology (Dr. Xiaoqing Li, Dr. Hejian Xiong, Hanwen Fan, and Dr. Xiaoqian Ge), neuro-oncology (Dr. Xiaofei Gao, Dr. Vamsidhara Vemireddy, and Dr. Elizabeth Maher, UT Southwestern Medical Center), small animal imaging (Ryan Magolis, Dr. Junjie Li, and Dr. Kenneth Hyot, the University of Texas at Dallas), and vascular biology (Dr. Monica Giannotta, FIRC Institute of Molecular Oncology Foundation).
Selected references
- van Tellingen, O., Yetkin-Arik, B., de Gooijer, M. C., Wesseling, P., Wurdinger, T., & de Vries, H. E. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Resist. Updat. 19, 1-12 (2015).
- Neftel, C., Laffy, J., Filbin, M. G., Hara, T., Shore, M. E., Rahme, G. J., Richman, A. R., Silverbush, D., Shaw, M. L., Hebert, C. M., Dewitt, J., Gritsch, S., Perez, E. M., Gonzalez-Castro, L. N., Lan, X., Druck, N., Rodman, C., Dionne, D., Kaplan, A., Bertalan, M. S., Small, J., Pelton, K., Becker, S., Bonal, D., Nguyen, Q. D., Servis, R. L., Fung, J. M., Mylvaganam, R., Mayr, L., Gojo, J., Haberler, C., Geyeregger, R., Czech, T., Slavc, I., Nahed, B. V., Curry, W. T., Carter, B. S., Wakimoto, H., Brastianos, P. K., Batchelor, T. T., Stemmer-Rachamimov A, Martinez-Lage, M., Frosch, M. P., Stamenkovic, I., Riggi, N., Rheinbay, E., Monje, M., Rozenblatt-Rosen, O., Cahill, D. P., Patel, A. P., Hunter, T., Verma, I. M., Ligon, K. L., Louis, D. N., Regev, A., Bernstein, B. E., Tirosh, I., & Suvà, M. L. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 178(4):835-849.e21 (2019).
- Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K.Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Rev. Clin. Oncol. 15, 325–340 (2018).
- Wu, D., Chen, Q., Chen, X. Han, F., Chen, Z., & Wang, Y. The blood–brain barrier: structure, regulation, and drug delivery. Transduct. Target. Ther.8, 217 (2023).
- Sarkaria, J. N., Hu, L. S., Parney, I. F., Pafundi, D. H., Brinkmann, D. H., Laack, N. N., Giannini, C., Burns, T. C., Kizilbash, S. H., Laramy, J. K., Swanson, K. R., Kaufmann, T. J., Brown, P. D., Agar, N. Y. R., Galanis, E., Buckner, J. C., & Elmquist, W. F. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Oncol.20, 184–191 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in