How to Structure a Clinical Case Report
Published in Biomedical Research and Education
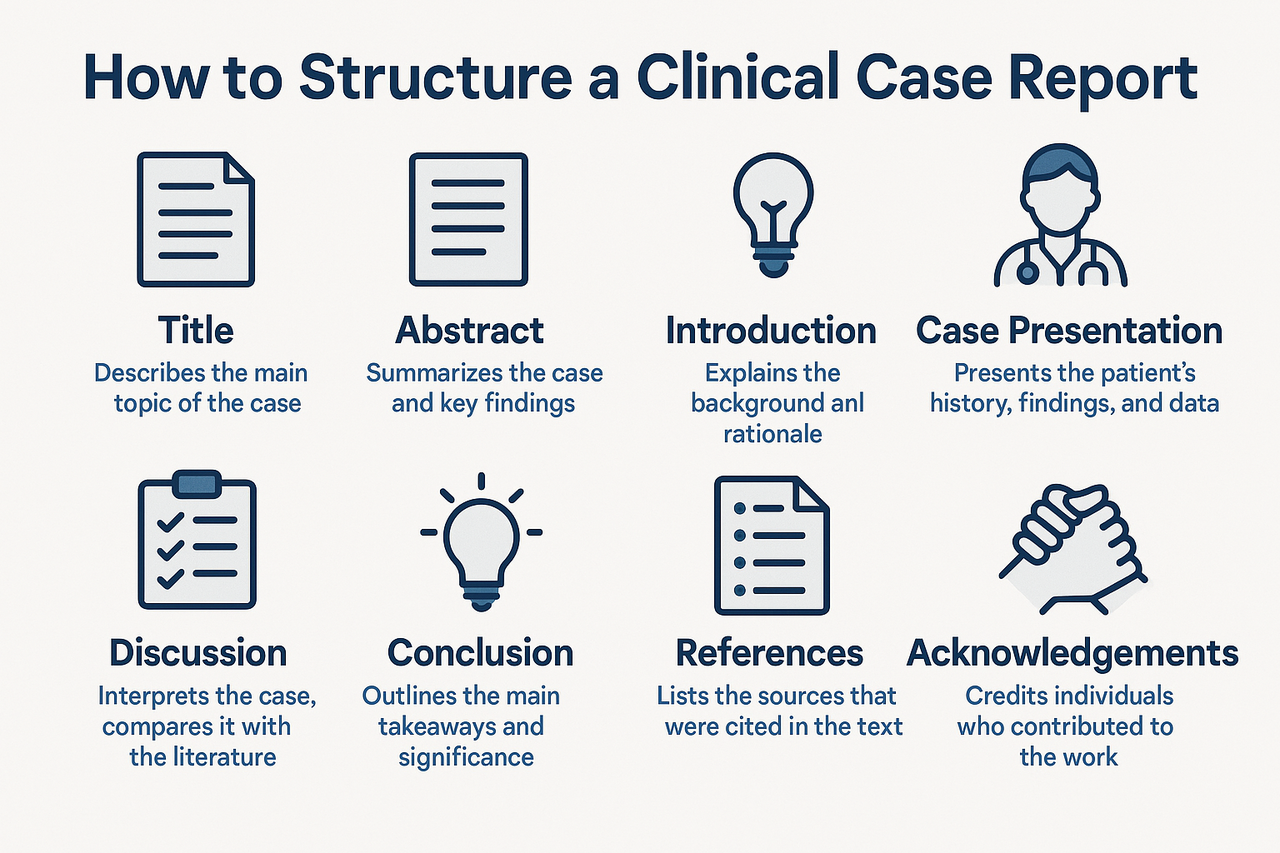
Clinical case reports are an essential component of medical literature, offering insights into rare diseases, atypical presentations, unexpected treatment responses, or novel complications. Despite their concise format, case reports must be structured with academic precision to ensure clarity, reproducibility, and impact. For medical students, trainees, and even experienced clinicians, understanding how to systematically present a case is crucial for successful publication.
Begin with a clear and specific title that captures the essence of the case. It should include the diagnosis or clinical entity and highlight its uniqueness. Avoid vague titles such as “A rare case”; instead, aim for informative ones like “Metastatic Ameloblastoma Mimicking Thyroid Carcinoma: A Diagnostic Dilemma,” which immediately conveys the clinical intrigue. Next, the abstract should briefly summarize the case using a structured format typically including background, case presentation, and conclusion. This section should highlight the clinical relevance in a way that attracts the reader's interest.
Following the abstract, include keywords (3 to 6), ideally aligned with MeSH terms, to optimize searchability and indexing. Then, begin the introduction by outlining the clinical background and explaining why the case is significant. This section should cite relevant literature and establish the context for the report, but remain brief usually no more than one to two paragraphs.
The case presentation is the core of the report and should be written with chronological clarity. Begin with the patient’s demographic details (age, sex, and pertinent history), followed by a detailed description of clinical symptoms and signs. Diagnostic findings, laboratory results, imaging, histopathology, and molecular testing should be reported in sequence, using figures and tables where appropriate. Discuss how the final diagnosis was reached and elaborate on the treatment plan and clinical outcomes. If follow-up data are available, they should be included to provide insight into the prognosis or response to treatment.
The discussion section interprets the case in the context of existing knowledge. This is where you compare your findings with those reported in the literature, highlight unusual aspects of the case, and discuss relevant pathophysiological mechanisms, diagnostic challenges, or therapeutic strategies. You may also reflect on limitations, such as lack of follow-up or advanced molecular testing. Conclude the discussion with the key clinical lesson learned from the case.
A brief conclusion should follow, restating the significance of the case and its implications for clinical practice. This section should not introduce any new information and is usually no longer than a few sentences. Importantly, all case reports must include a patient consent statement to confirm ethical compliance. For example, you may write, “Written informed consent was obtained from the patient for publication of this case and accompanying images.”
Supporting figures and tables (e.g., radiographs, histology images, flowcharts) enhance the clarity and educational value of the case. Ensure that all images are anonymized to protect patient identity and are accompanied by descriptive legends. Lastly, the references should be accurate, up-to-date, and formatted according to the target journal’s guidelines, typically using the Vancouver or APA style.
In summary, a well-structured clinical case report includes a clear title, structured abstract, succinct introduction, detailed case narrative, insightful discussion, and ethical compliance. By adhering to guidelines such as CARE or ICMJE, authors can ensure their reports are not only publishable but also meaningful contributions to clinical education and practice. Whether documenting a diagnostic puzzle, a novel treatment response, or a rare manifestation of a common disease, a case report offers a unique opportunity to share clinical wisdom with the broader medical community.
| Section | Purpose | Professional Writing Guidelines |
|---|---|---|
| Title | Clearly conveys the diagnosis and clinical uniqueness of the case. | Be concise and specific. Avoid vague terms. Highlight diagnostic dilemma, rarity, or unusual presentation. |
| Abstract | Provides a brief summary of the case, including context, key findings, and clinical impact. | Use a structured format: Background, Case Presentation, and Conclusion. Limit to 150–250 words. |
| Keywords | Enhance searchability and indexing in databases. | Select 3–6 relevant keywords, preferably using MeSH terms. |
| Introduction | Establishes the clinical background and rationale for reporting the case. | Summarize known information and emphasize what makes this case novel or educational. Cite relevant literature. |
| Case Presentation | Detailed clinical narrative covering history, diagnostics, treatment, and outcomes. | Maintain chronological order. Use clear headings. Ensure patient confidentiality and de-identify all data. |
| Discussion | Interprets the case in the context of current medical knowledge and literature. | Compare findings with similar reports. Discuss pathophysiology, diagnostic reasoning, management decisions, and gaps. |
| Conclusion | Summarizes key clinical insights or lessons learned. | Be succinct. Reinforce the educational or research value. Avoid introducing new information. |
| Patient Consent | Confirms ethical compliance and permission to publish patient data. | Include a formal statement: “Informed consent was obtained for publication.” |
| References | Supports the scientific basis of the report. | Use updated, peer-reviewed sources. Format according to journal style (e.g., Vancouver). |
| Figures and Tables | Visual representation of findings (e.g., imaging, histopathology, timelines). | Use high-resolution images. Anonymize patient data. Include descriptive legends and proper labeling. |



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in