How will today’s patent law affect tomorrow’s innovation in the areas of biomarkers; nature-based products; diagnostics; and algorithms, big data and AI?
Published in Bioengineering & Biotechnology
Summary
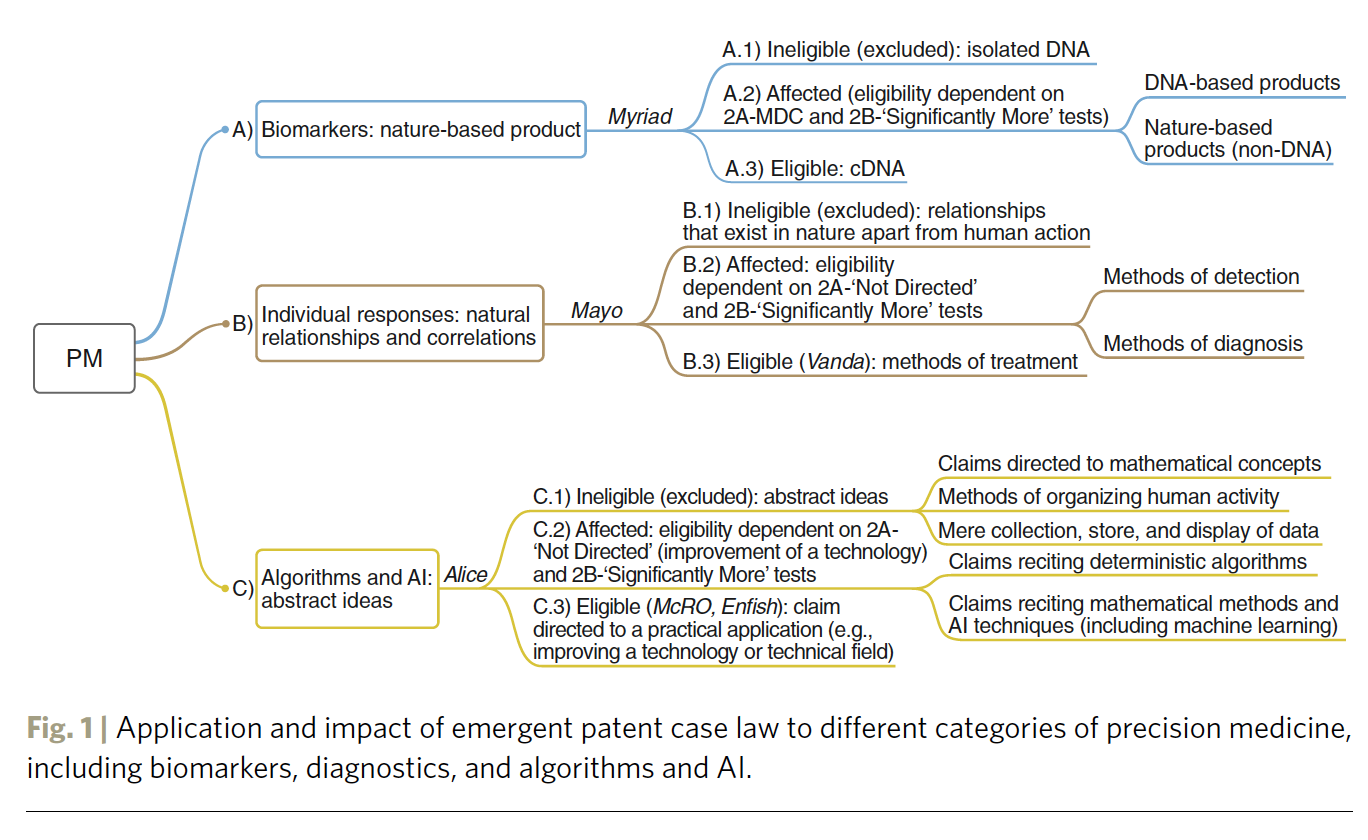
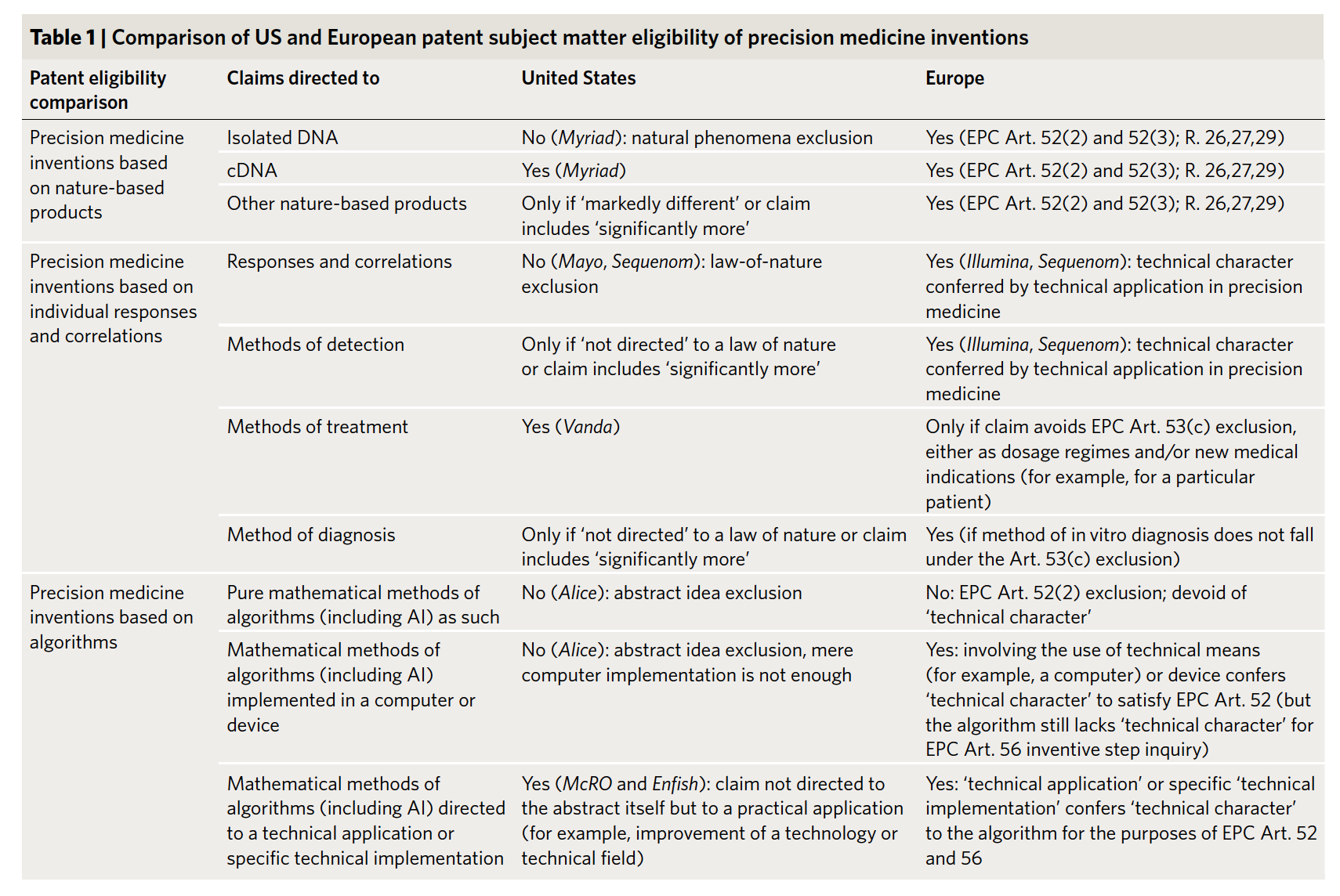
Precision medicine has stirred the excitement of patients, physicians, industry, entrepreneurs, and investors — it is already a market that is worth tens of billions of dollars with very rapid growth expected. But the future of precision medicine heavily depends on the intellectual property frameworks that apply to its various forms. In order to chart this future, in this Nature Biotechnology paper we compare recent patent case law in the US and Europe in three areas of precision medicine: (1) biomarkers and nature-based products; (2) diagnostics; and (3) algorithms, big data, and AI. Our overarching goal is to explain how today’s law will affect tomorrow’s innovation.
Behind the Article
The idea behind the paper originated at the 2018 CeBIL Annual Symposium that took place at Christ's College (University of Cambridge, UK) on September 7th, 2018 focused on Precision Medicine, Artificial Intelligence, and the Law (PMAIL). Following the presentation on the impacts of the Myriad, Mayo, and Alice decisions based on our previous evidence-based patent studies published in Nature Biotechnology, the co-authors started a discussion about the need for a paper that would incorporate: 1) the key US and European decisions affecting precision medicine over the last 6 years, 2) the results from empirical studies analyzing the impact of these decisions, 3) the latest 2019 Examination Guidance of the USPTO and EPO, and 4) a comparative study of the US and European patent subject matter eligibility for precision medicine inventions. Additionally, the paper would address the three key tenets of precision medicine (biomarkers, diagnostics, and algorithms/AI).

Prof. Mateo Aboy’s talk on Precision Medicine, AI and the Law at the 2018 CeBIL Symposium
The Team
The paper is co-authored by a multinational team of scholars from the University of Cambridge (LML), Harvard University (Petrie-Flom) and University of Copenhagen (CeBIL). The perspectives from the US, UK and Europe, as well as the multidisciplinary nature of the team were key to make the paper a reality. The co-authors are core partners of CeBIL.
Our Hope
Like all authors, we tried to write the go-to-paper in the field which answers cutting edge questions on IP and precision medicine in a single place. We hope it will be of significant interest to the readership of Nature, including scientists, physicians, legal scholars, patent practitioners, entrepreneurs, investors and corporate strategists. We know from experience that, inevitably, there will be further policy developments, possibly even substantial changes. Indeed the constancy of change was a theme in our paper. But we saw this horizon and hope that the paper will be consulted as a panoptic piece for many years to come.
Funding
The research was supported, in part, by a Novo Nordisk Foundation grant for a scientifically independent Collaborative Research Programme in Biomedical Innovation Law (Grant No. NNF17SA027784).
Follow the Topic
-
Nature Biotechnology

A monthly journal covering the science and business of biotechnology, with new concepts in technology/methodology of relevance to the biological, biomedical, agricultural and environmental sciences.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in