Humans, sea slugs, kidney cells: we all learn the same way
Published in Neuroscience and Behavioural Sciences & Psychology
We are used to thinking that we learn with our brains. But our new study shows that all cells in our body — even kidney cells — can learn. What’s more, their learning follows the same rules and uses the same means as the more familiar learning, the kind that produces changes in neurons, and modifies future behavior.
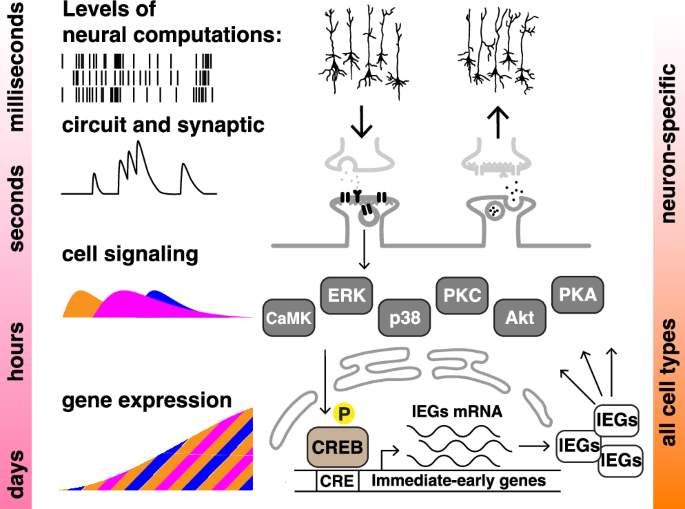
What could those other, non-neural cells be learning? Consider first what neurons are learning, exactly. Most neurons that store our memories do not interact directly with the outside world. They receive information in the form of chemical patterns spread in time: which of their synapses are being bombarded by which neurotransmitters of other neurons, with what intensity and in what order. To a neuron, human experience is a convoluted pattern of chemicals. The memory is the change that this pattern produces, a change that, in the future, adjusts how the neuron acts. There can be short-term changes and long-term changes, each caused by some distinct molecular mechanism. Some patterns of neurotransmitters — a rapid burst of glutamate, for example — cause changes in ion channels which persist for a short while. More prolonged patterns — say, repeated bursts over an hour — turn on “memory genes”, whose products then go on to modify the neuron in deeper and more prolonged ways. Yet more prolonged patterns, such as those associated with long-term lifestyle factors, can cause even deeper changes, such as epigenetic modifications.
So learning, from the point of view of a neuron, is converting time patterns of chemicals into internal changes that persist.
Other cells also receive patterns of chemicals: nutrients, hormones, signaling molecules from their neighbors. They also convert them into cellular changes that persist: molecular modifications, “memory genes”, epigenetic marks. These changes alter the way those cells act. For example, insulin-producing cells have short-term memory of glucose: two 20-minute pulses of glucose separated by 20 minutes of perfusion produce a two-fold increase in the release of insulin during the second pulse compared to the first pulse. So it is not news that cells in the body can become modified in response to chemical signals.
The question is — is there enough information stored in those non-neuronal memories to even call them memories?
Another way to ask the same question: how fine a pattern can a non-neuron distinguish in the timing of chemicals it receives?
This was the question that we set out to ask. Can a non-neural cell tell the difference between patterns of chemicals that are distinct on a timescale of only a few minutes, and convert the correct pattern into a change that persists? This is typical behavior for a neuron, but it’s not usually attributed to other kinds of cells. We engineered two separate cell lines, one from a neuroblastoma and one from kidney, to produce a glowing protein any time their “memory gene” was turned on — we used the same gene as neurons use. We then gave those cells pulses of chemicals, which we picked to activate the same parts of the cells that are usually activated in neurons during learning.
It turned out that our non-neural cells could tell apart very specific patterns. First, they could count — at least to four. A three-minute pulse did turn on the “memory gene”, but only for a short period — the change died down after a couple of hours. Four of those pulses spaced by 10 minutes produced a change that was only slightly stronger initially, but it persisted almost unchanged for at least 24 hours.
This is exactly how memory works: repetition not only boosts the immediate strength of the memory, but also slows down forgetting. This was famously first demonstrated by Hermann Ebbinghaus, pioneering 19th century researcher who systematically studied lists of nonsense syllables and meticulously documented his own recall over time.
Ebbinghaus established another property of memory: the spacing effect. Learning crammed in one sitting is less effective than the same amount of learning spread over multiple sessions spaced in time. This effect has since proven to be one of the most unshakeable properties of memory in many different animals. Our interest in it stems from our previous studies in the sea slug Aplysia californica, which also learns better from spaced training sessions than from a crammed one. Even cultures of neurons in Petri dishes show the spacing effect: they respond more strongly to several excitatory pulses spaced in time than to a single long pulse of the same total length.

But no one has ever seen this spacing effect outside the nervous system. Telling apart a sequence of spaced pulses from a single long pulse, when the two patterns are not distinct in overall amount of stimulation and only differ in a few minutes of timing, just seems like too sophisticated a pattern recognition to be done by a non-neural cell. Could it be that our humble kidney cells can do that, too?
It turns out they can. When the cells received a single prolonged pulse equivalent in length to the spaced pulses, they turned on the “memory gene” less strongly. This “crammed” pulse also caused a weaker activation of several cellular components known to be involved in memory formation, showing that the difference between the two patterns is detected similarly to how neurons do it.
So non-neural cells are a lot smarter than we give them credit. There is, we now understand, a deep continuity of learning across all cell types, neurons or non-neurons, human or sea slug. All this learning follows the same rules, such as the spacing effect and the slowdown of forgetting with repetition, even though neither of those rules are a priori features of learning or just any biochemical system. In digital computers, learning does not show a spacing effect. In enzymology, a greater buildup of a product generally results in faster, not slower decay. But in biological learning — whether it is done by neurons or other cells — the principles established by Ebbinghaus continue to prove universal.
What this means is that in the future, we might need to consider not just our own memory, but the memories of all of our cells when we think about our body and well-being. If pancreatic cells can remember the patterns of food, and kidney cells can remember patterns of cellular signals, then the experiences of these cells — the patterning of our meals, the timing of exercise, the drug regimens — are just as much a part of our health as the experiences of our neurons.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in