Hydrogenation Reactions with Photochargeable Carbon Nitride
Published in Chemistry
It seems that storage of hydrogen in the form of separated electrons and protons is even greater challenge. True, unless we use a special vessel, or better to say a special medium that keeps e‒/H+ separately.
Upon illumination, various materials can ‘extract’ e‒/H+ from sacrificial agents and accumulate these species in the structure. We can say that in this process the material is photocharged. This research topic could be tracked back to 1980th, to for example electron storage in TiO2 nanoparticles described by Armin Henglein or possibly even earlier period. The topic gained new momentum in the past several years. In particular, a recent article from CataLight research center proves the trend.
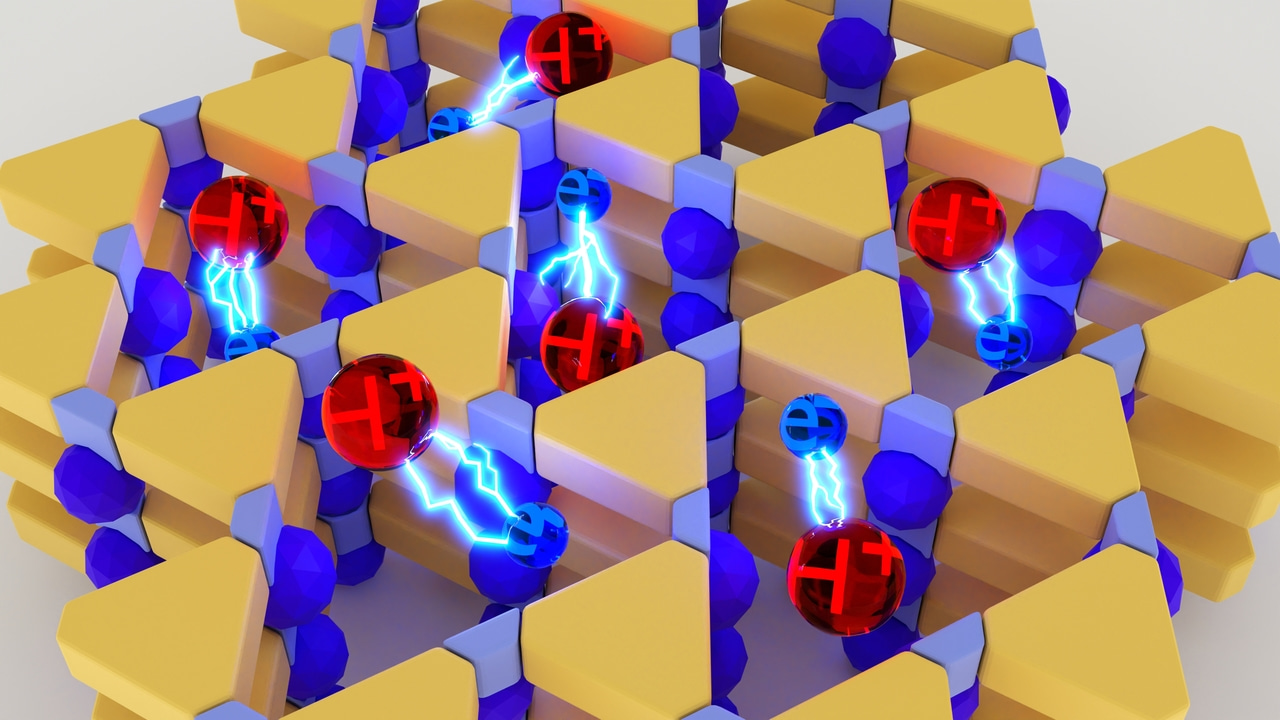
Conjugated microporous structure of potassium poly(heptazine imide) (K-PHI) is perfectly suitable for photocharging. Akin a battery, in this case electrons and protons travel together inside the micropores of the material until up to 1 mmol of electrons is accumulated per gram of the material. K-PHI is soaked with electrons! As a result, the color changes from yellow, typical for uncharged carbon nitrides, to dark green (see figure below).
Earlier photocharged carbon nitrides were applied in H2 evolution. As e‒/H+ do not recombine spontaneously when stored in the material, a co-catalyst is required to trigger evolution of H2.
The project that we shaped into a research article started with the question: “In what other reactions beyond evolution of H2 can we use electrons and protons stored in K-PHI(e‒/H+)?”
We definitely can hydrogenate unsaturated bonds in organic molecules via proton-coupled electron transfer (PCET)! In our recent project, we applied photocharged K-PHI(e‒/H+) particles in reduction of arylhalides to hydrocarbons.
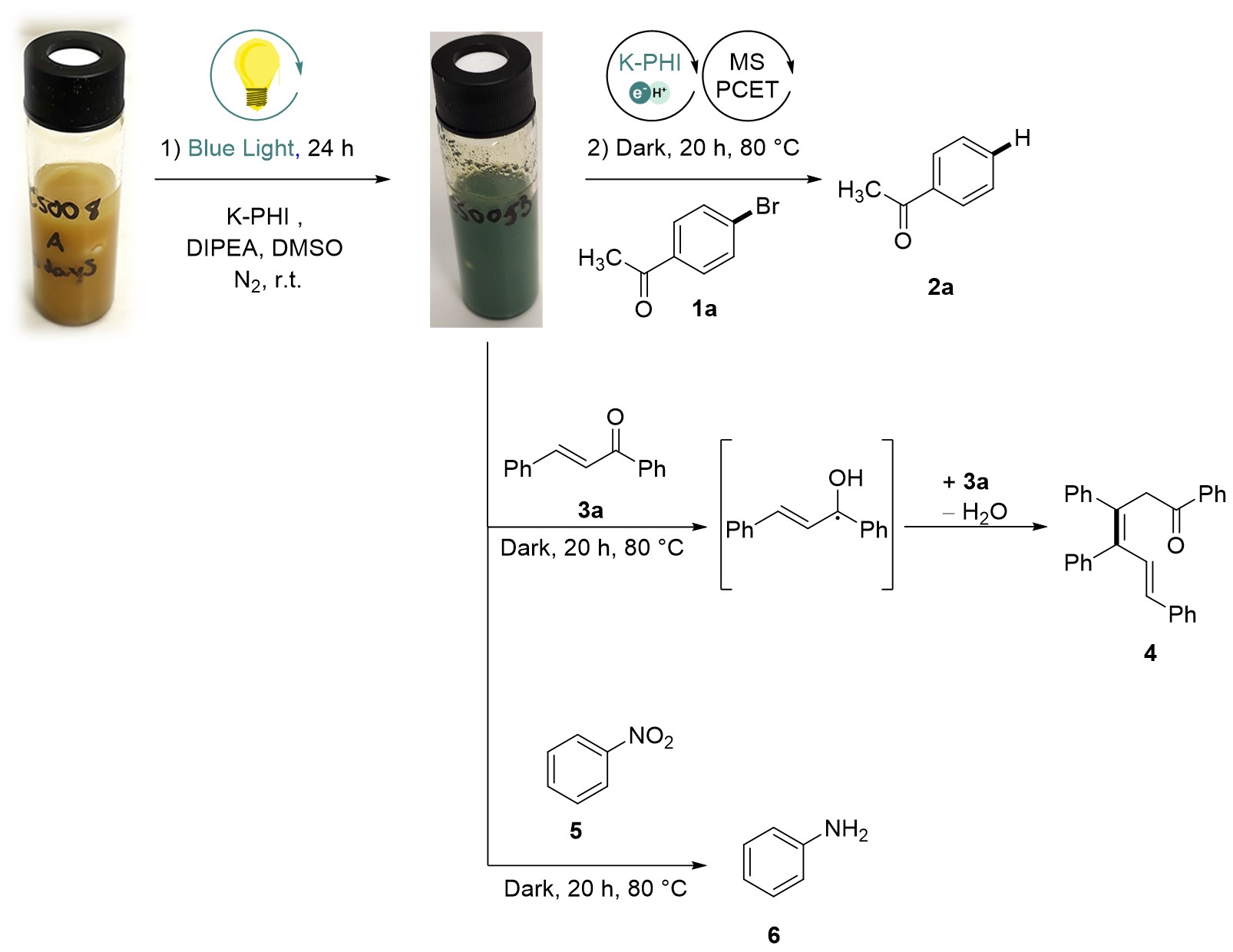
Photocharged K-PHI(e‒/H+) particles also reduce nitrocompounds to anilines. However, the most special reaction is PCET to enone that gives a ketyl radical. Dimerization of ketyl radicals followed by ellimination of water produces hexadienone – a structure that is hardly accessible in a such simple way.
In this project, K-PHI particles serve as a reductant in organic synthesis that can be charged and recharged by light. Read the open access article in Exploration.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in