ICU patient-on-a-chip emulating orchestration of mast cells and cerebral organoids in neuroinflammation
Published in Healthcare & Nursing, Neuroscience, and Pharmacy & Pharmacology
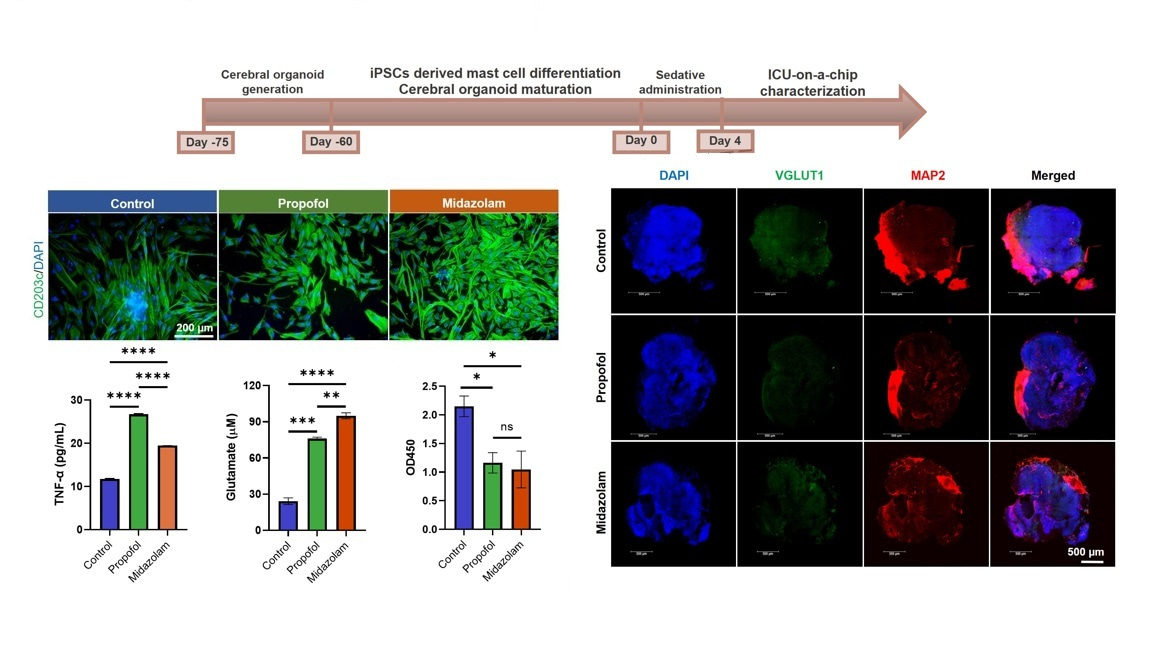
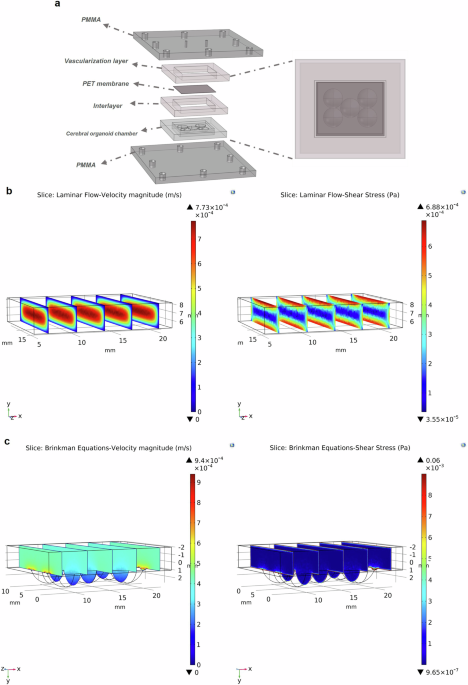
In Intensive Care Units (ICUs), sedatives like propofol and midazolam are frequently used to manage patients who require prolonged sedation. While these drugs are essential for maintaining comfort and controlling anxiety in critically ill patients, the effects they have on the brain and the body’s immune response remain underexplored. Understanding these impacts is crucial, as prolonged sedation may result in long-term complications, including cognitive dysfunction and neuroinflammation. Traditional research into sedative effects often involves animal models, but these do not always fully replicate human responses. To address these gaps, we developed a cutting-edge ICU patient-on-a-chip platform to study the effects of these sedatives on brain tissue and the blood-brain barrier. This platform features a 3D co-culture of human-induced pluripotent stem cells (hiPSCs)-derived mast cells and cerebral organoids, supported by a membrane populated with human cerebral microvascular endothelial cells (hCMEC/D3), which form the blood-brain barrier. The platform allows us to infuse sedatives like propofol and midazolam for four days, mimicking the prolonged exposure seen in ICU settings. By monitoring the neurotoxicity and immune responses, we gain invaluable insights into the potential risks of these sedatives, particularly on brain tissue and the integrity of the blood-brain barrier.
Key findings with respect to propofol vs. midazolam
Our results indicate that both propofol and midazolam activate distinct cellular pathways and result in different neuroinflammatory and neurotoxic responses.
Effects of propofol on mast cells and inflammation: Propofol administration led to the activation of CD40 and TNF-α expression in mast cells. This is a marker of immune activation, which plays a role in inflammation. Additionally, elevated expression of AIF1 in microglia and GFAP/S100B/OLIG2/MBP in macroglia suggests that Propofol may trigger both immune cells and glial cells in the brain.
Neuroinflammation: Gene expression markers like NOS2, CD80, CD40, CD68, IL6, and TNF-α were upregulated in cerebral organoids, indicating a pro-inflammatory response. This inflammation was associated with higher expression of GJB1 (a protein related to cell communication) and an increase in GABA-A and NMDAR1 receptors, which are implicated in neurotransmission.
Effects of midazolam on mast cell proliferation and BBB disruption: Unlike propofol, midazolam predominantly activated mast cells, increasing the expression of CD40 and CD203c+, markers of mast cell activation and proliferation. Moreover, it compromised the integrity of the blood-brain barrier, as indicated by reduced TEER values and barrier disruption.
Microglia and glial cells: Midazolam also triggered an increase in CD11b+ microglia and GFAP expression in macroglia. This suggests that midazolam may promote microglial activation and glial cell differentiation, which can contribute to neuroinflammation and neurotoxicity.
Neurotoxic and inflammatory responses: Additionally, we observed increased glutamate-related neurotoxicity and upregulated genes such as IL1B, IFNG, IFNA1, and IL6, which are known to mediate inflammation. In contrast to propofol, midazolam exposure led to lower expression of GABA-A and NMDAR1 receptors, indicating a shift in neurotransmitter signaling.
Implications of the findings
Our findings suggest that different sedative drugs activate distinct cellular pathways that could lead to varying degrees of neuroinflammation, neurotoxicity, and disruption of the blood-brain barrier. These differences are crucial to consider, especially in the context of long-term sedation in ICU patients. Propofol appears to trigger more general neuroinflammation, affecting both glial cells and immune responses. In contrast, midazolam seems to have a more targeted effect on mast cells, with significant consequences for the blood-brain barrier's integrity and neurotoxicity, potentially making it riskier for prolonged use in certain ICU patients. The ICU patient-on-a-chip platform offers a more precise and human-relevant method to study the effects of these sedatives, providing insights that may lead to safer sedation protocols in critical care. By better understanding how different sedatives impact brain tissue and the immune system, we can optimize their use to reduce the risk of long-term complications like cognitive dysfunction and BBB disruption.
Conclusion
This study highlights the potential of humanized microphysiological platforms in advancing our understanding of sedative effects in critically ill patients. By examining the complex interactions between drugs, immune cells, and brain tissue in a controlled environment, we can better assess the risks and benefits of sedatives like propofol and midazolam. With continued research, this approach could pave the way for safer, more effective sedation strategies in ICUs, ultimately improving patient outcomes and reducing the long-term risks associated with sedative use.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in