Imaging Cu₂O nanocube hollowing in solution by quantitative in situ X-ray ptychography
Published in Materials
Hollow nanoparticles with sizes in the range of several hundred nanometers have widespread application potential. They are often utilized to form composite materials for high-performance electrodes in lithium ion batteries, for (photo-) catalytic energy production and as sensors. To reach the desired functionality and high performance, it is decisive to achieve precise control over the structure and shape of the nanoparticles during their growth. However, understanding and manipulating the course of the underlying morphological changes remains a major challenge, which is mainly due to the lack of experimental methods that can observe the dynamics of chemical processes in situ in a bulk solution under relevant conditions, such as high temperature and pressure. Visual insights into the formation dynamics of nanomaterials are thus rare. Even though liquid-cell transmission electron microscopy (TEM) can provide spatial resolution down to the atomic scale, its applicability to solution-based processes is limited, since it requires thin reactors with small volumes which can alter the kinetics of chemical reactions.
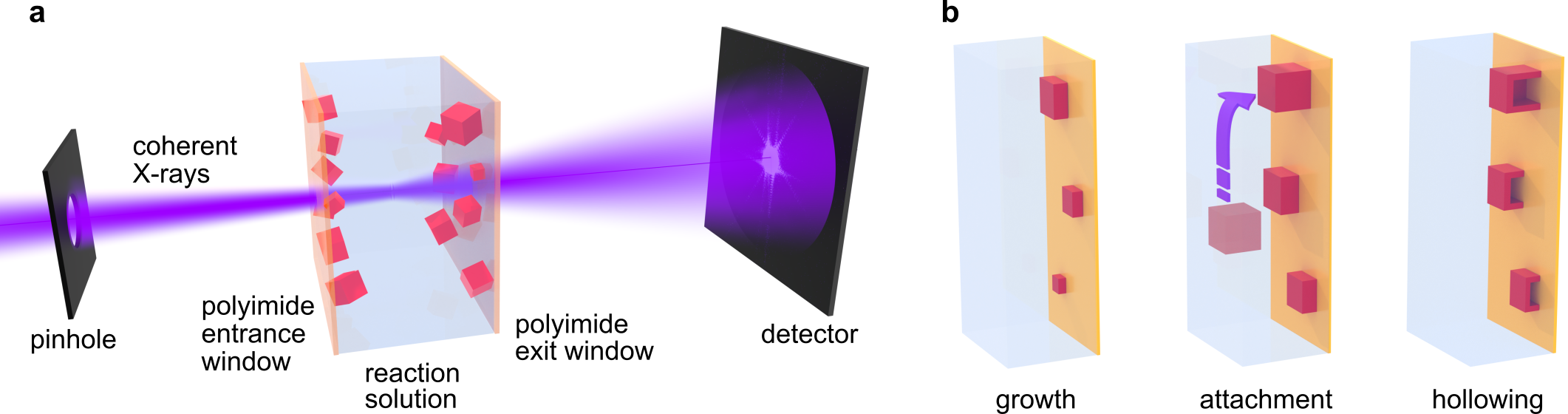
Figure 1. Illustration of the in situ microscopy experiment. a For ptychographic imaging, the reactor containing growing Cu2O nanocubes is raster-scanned through a focused coherent X-ray beam. Diffraction patterns are recorded in the far field for image reconstruction. b Illustration of the growth and hollowing of substrate-bound nanocubes.
Adapted from the original publication licensed under the CC BY 4.0 International License.
Recently, a research team led by Prof. Dorota Koziej at the University of Hamburg, Germany, and the Cluster of Excellence “CUI: Advanced Imaging of Matter”, also involving researchers from the Deutsches Elektronen-Synchrotron DESY in Germany, the University of Cambridge in the United Kingdom, the Sao Paulo State University in Brazil, and the Paul Scherrer Institute in Switzerland, demonstrated the use of X-ray ptychography for in-solution imaging of nanoparticles, overcoming the limitations of in situ TEM (Figure 1). They observed the growth and hollowing of Cu2O nanocubes in solution inside a chemical reactor with a thickness of 1 mm (Video 1). The findings have been published in Nature Communications in an article entitled “Imaging Cu2O nanocube hollowing in solution by quantitative in situ X-ray ptychography”.
Multi-sliced ptychography enables imaging in thick chemical reactors
X-ray ptychography, which is a scanning-based coherent diffraction imaging technique, can be used for in situ nano-imaging of the shape evolution of nanomaterials in solution. To this end, the team developed a liquid reactor that provides the necessary mechanical and thermal stability (Figure 2). The Cu2O nanocubes nucleate mostly heterogeneously on the walls of the reactor, which immobilizes them for long-term imaging. Ptychography allows to reconstruct separate 2D images of particles growing on the entrance and exit windows of the reactor by applying the multi-slice model. This way, the researchers tracked the growth and hollowing of individual nanocubes at a spatial resolution as high as 66 nm and with a time resolution of 22 min.

Figure 2. Photographs of the in situ reactor. a Flat liquid container with two polyimide windows containing the reaction solution. b The reactor mounted at the Ptychographic Nano-Analytical Microscopy (PtyNAMi) at the nanoprobe endstation of beamline P06 at the synchrotron source PETRA III at DESY.
Credit: Martin Seyrich / DESY.
Quantitative phase images reveal the shape of nanocubes in 3D
Interestingly, new nanocubes successively appeared in the images in addition to those particles that nucleated on the reactor windows and were thus visible from the beginning of the reaction (c.f. Video 1). The new particles must have nucleated homogeneously in solution and attached to the windows at some point during their growth process. To investigate the influence of the nucleation behavior on the shape of the final nanocubes, the researchers made use of the quantitative nature of the ptychographic images, which contain the local phase shift induced by the sample. The phase shift gives access to physical parameters such as the area density or, given that the composition of the sample is known, the thickness of the material.
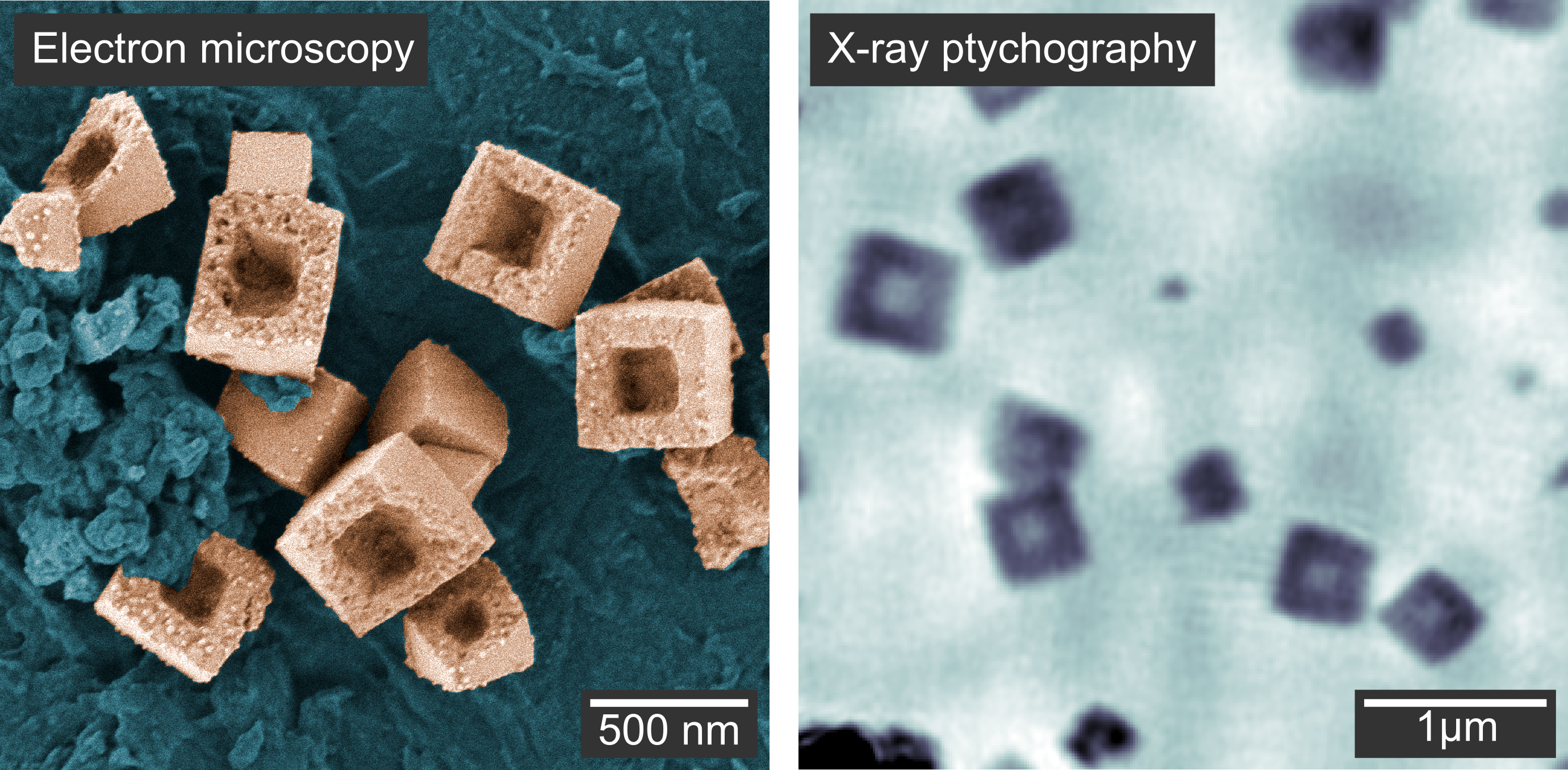
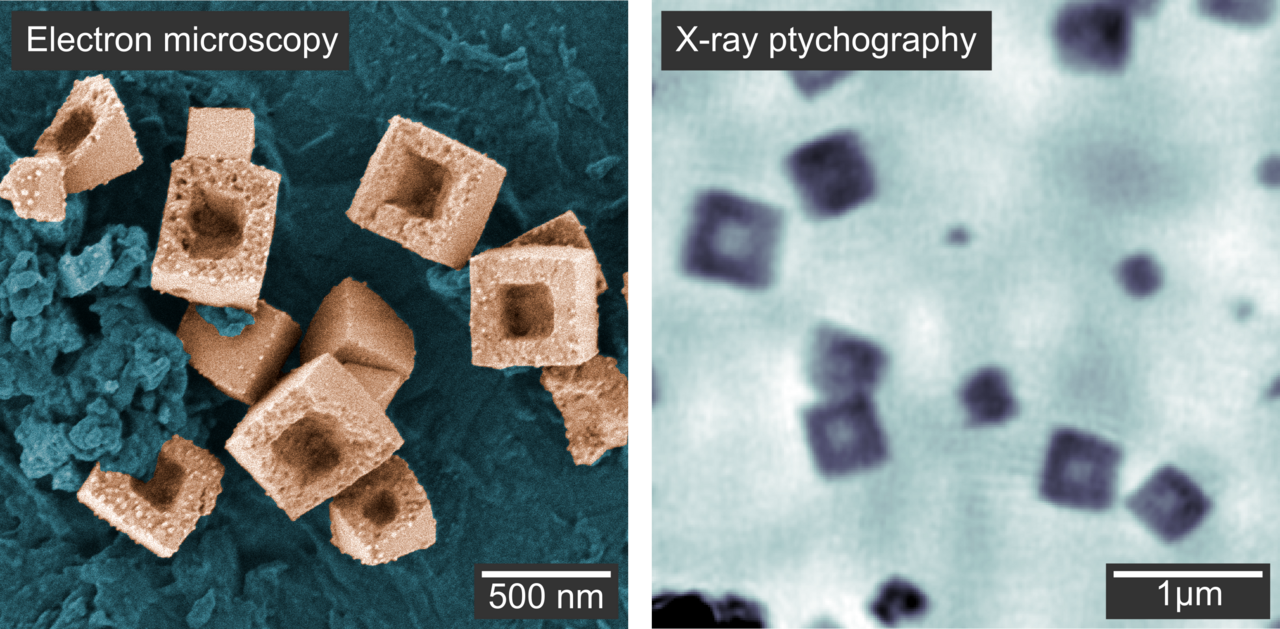
Figure 3. a Scanning electron microscopy image of the hollow copper nanocubes outside the chemical reactor. b Image of the hollow nanocubes inside the chemical reactor using X-ray ptychography. Although this image appears more blurred, ptychography has the great advantage of taking images in real time within the liquid reactor.
Adapted from the original publication licensed under the CC BY 4.0 International License.
In this way, they calculated the thickness of the nanocubes, allowing them to infer the full growth and hollowing process in 3D: particles growing heterogeneously on the reactor windows showed a flat shape with an aspect ratio of 0.5, while particles in solution took a cubic shape. Thus, the window material hindered the growth of the particles in the direction perpendicular to the window. In a second reaction stage, the nanocubes were reduced to metallic copper in a solid-state process. Voids formed close to the interface between the nanocubes and the window material and expanded toward the particle surface, resulting in hollow nanocubes (Figure 3).
The study demonstrates how imaging in solution with hard X-ray ptychography enables a direct visualization of the complex transformations of shape and size at the nanoscale. The method can be applied to a wide range of materials and reaction conditions, complementing the capabilities of liquid-cell TEM. Such rare visual insights into structural changes in solution are important to deepen our understanding of the origins of nanomaterials morphology, which is a key factor for the future design of highly active catalysts and functional devices.
Video 1. Growth and hollowing of Cu2O nanocuboids imaged in situ with X-ray ptychography.
Adapted from the original publication licensed under the CC BY 4.0 International License.
Reference: L. Grote, M. Seyrich, R. Döhrmann, S. Y. Harouna-Mayer, F. Mancini, E. Kaziukenas, I. Fernandez-Cuesta, C. A. Zito, O. Vasylieva, F. Wittwer, M. Odstrčzil, N. Mogos, M. Landmann, C. G. Schroer, and D. Koziej, Imaging Cu2O nanocube hollowing in solution by quantitative in situ X-ray ptychography, Nature Communications 13, 4971 (2022), DOI: 10.1038/s41467-022-32373-2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in