
When considering bioimplants, one of the primary concerns is the foreign body response (FBR)—an immune-mediated reaction to foreign or synthetic materials. This response begins with protein adsorption and ultimately leads to the encapsulation of the implant by collagen and fibrotic tissue. Beyond posing risks to patients, FBR presents significant challenges for bioelectronic implants, as the resulting fibrotic barrier impedes intimate contact between the device and surrounding tissue, hinders signal transmission, and thereby limits the functional lifespan of the implant (Fig. 1). While substantial efforts have been devoted to designing non-electronic biomaterials that suppress FBR, comparatively little attention has been given to developing FBR-resistant designs for electronic materials.
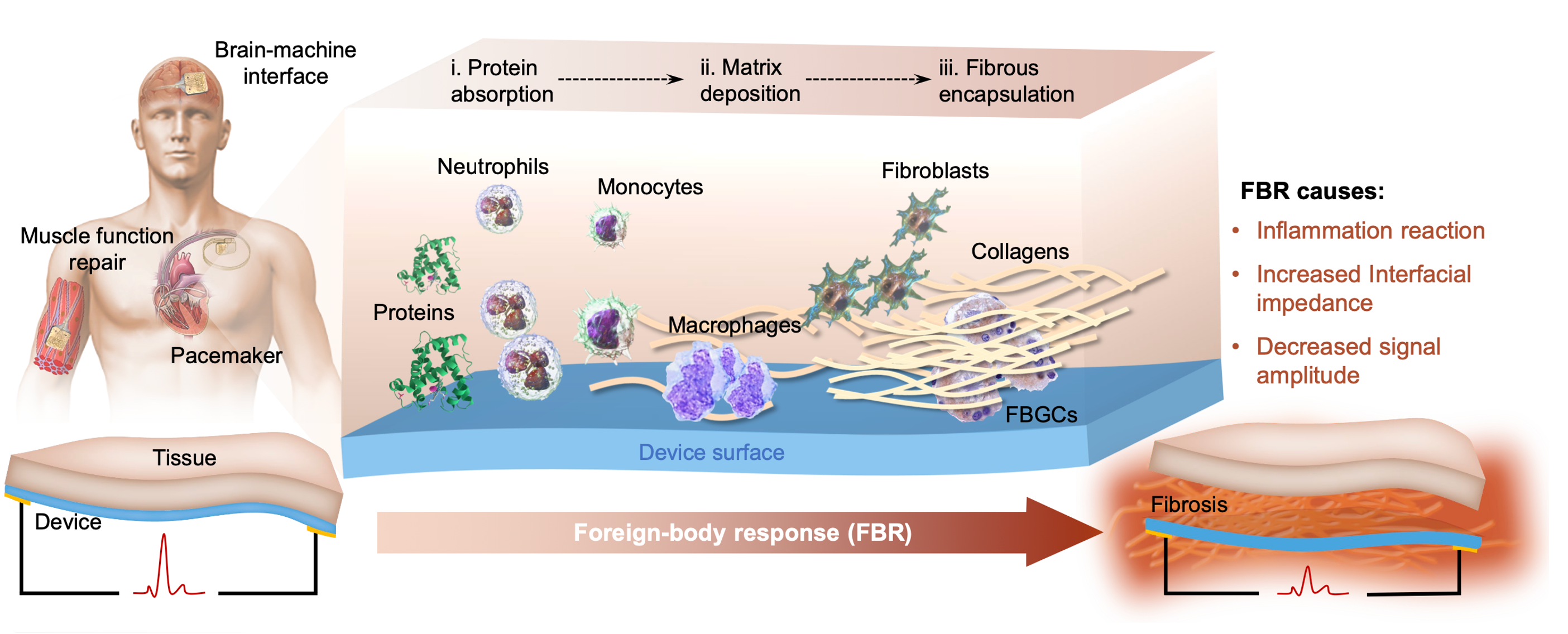
Figure 1. FBR processes on common types of implantable devices, and the negative influences
In particular, semiconductors—which have shown significant potential in various bioelectronic devices—need to be exposed at the device surface to enable signal transduction. While semiconducting polymers engineered with tissue-like mechanical properties can help mitigate FBR, substantial gaps remain in the development of immune-compatible semiconducting polymers. To address this unmet need, our recent work published in Nature Materials introduced a suite of molecular design strategies aimed at achieving intrinsic immune compatibility in semiconducting polymers.
We integrated selenophene into the polymer backbone as a replacement for thiophene, a strategy shown to simultaneously promote immune compatibility and maintain high electrical performance. In parallel, drawing inspiration from immunological studies highlighting the immunomodulatory functional groups, we incorporated such moieties (THP and TMO) into the side chains of semiconducting polymers to suppress FBR. Using the well-characterized p(g2T-T) as the starting polymer to implement these designs, we show a substantial reduction in FBR, as evidenced by a 68% decrease in collagen density around the implants (Fig. 2).
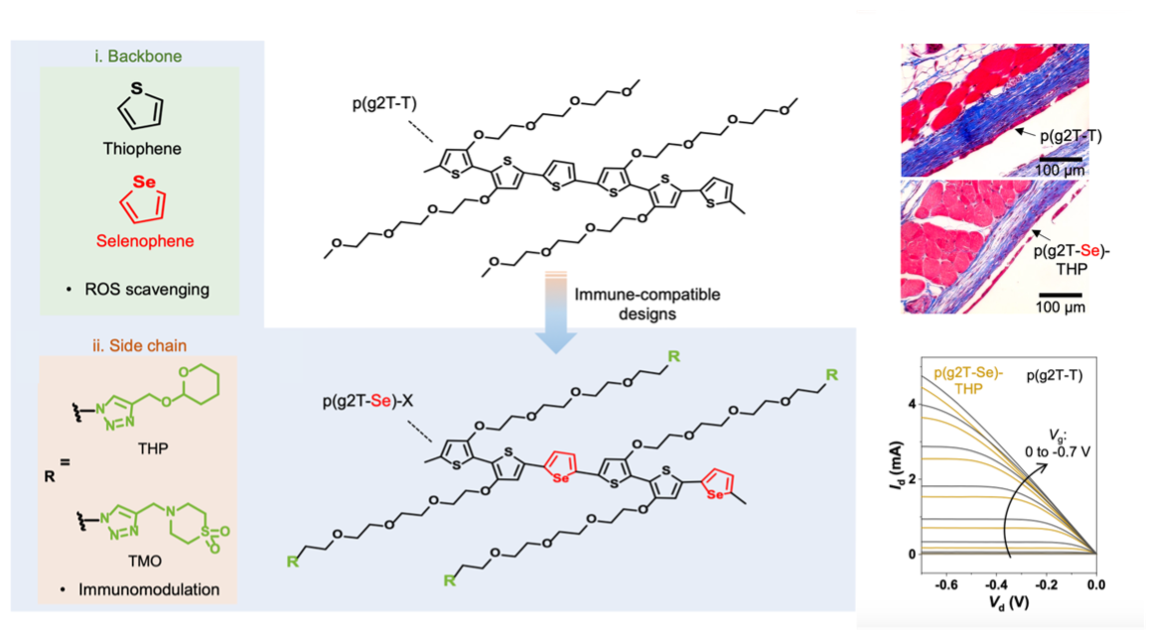
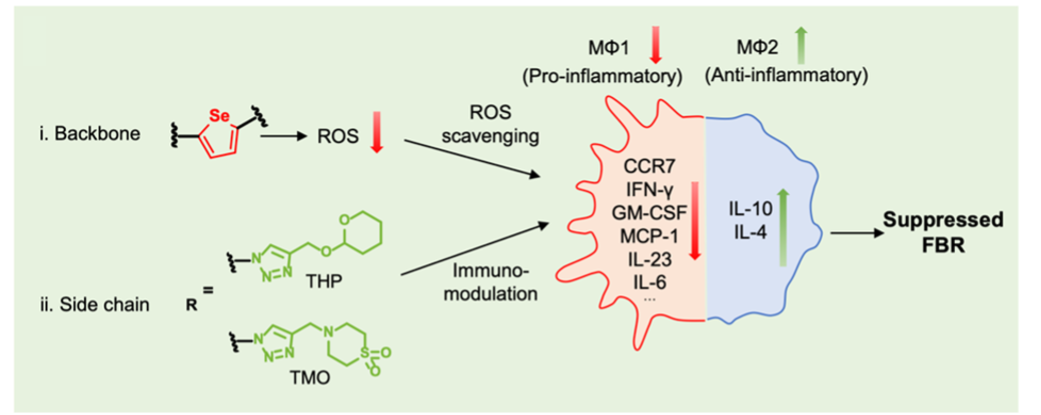
Previously, there had been no rigorous characterizations of FBR behaviors for semiconducting polymers. In order to provide a comprehensive understanding of the FBR-suppressing effects of our design, we carried out a series of systematic immunological assays. In brief, our two strategies were shown to downregulate pro-inflammatory biomarkers and upregulate anti-inflammatory ones, mitigating FBR by suppressing macrophage activation (Fig. 3). Furthermore, this study reveals the immunomodulatory potential of selenophene-based structures, highlighting a promising molecular design approach that leverages elements like selenide to construct conjugated units with intrinsic immune compatibility. The details can be found in our published paper.
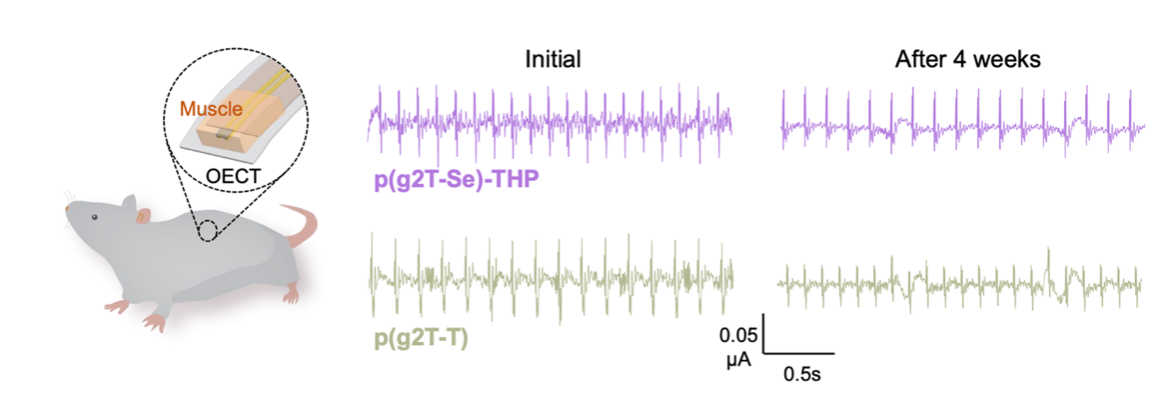
We demonstrated that the immune-compatible semiconductor designs maintained excellent electrical performance in organic electrochemical transistor (OECT) devices. Notably, the incorporation of a selenophene backbone resulted in higher charge carrier mobility that reaches 1.2 cm2V-1s-1, and also higher transconductance, compared to the conventional thiophene-based counterpart, underscoring the potential of selenide-containing backbone engineering. We further evaluated the in vivo chronic performance of our materials by comparing the current retention of implanted OECT devices over a three-month period. Taking a step further, we fabricated soft OECT devices based on our semiconducting polymers and successfully demonstrated the chronic use for the recording of electrocardiography (ECG) and electromyogram (EMG) signals in live mice. After four weeks of implantation, the immune-compatible designs maintained significantly higher signal amplitudes, highlighting their promise for chronic bioelectronic applications (Fig. 4).
We believe the versatility of these design principles for immune-compatible conjugated polymers would open up a broad platform for expanding into other functional categories, such as biochemical sensing, fluorescence imaging, and bio-stimulations. Integrating these strategies with low-modulus designs could further alleviate mechanical mismatch at the tissue interface. Beyond these immediate applications, conjugated polymers—owing to their chemically distinct structures—offer a unique opportunity for the systematic study of FBR. Insights gained from this study could drive the development of next-generation bioelectronic devices, paving the way for intrinsically immune-compatible, multifunctional and high-performing interfaces between electronics and biological tissue.
This work titled “Immune-compatible designs of semiconducting polymers for bioelectronics with suppressed foreign-body response” was published in the Nature Materials.
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in