Inducing pyrrole rearrangements for chromophore studies
Background
Molecular photoswitches can be used with light to control reversible movement at the nanoscale1. The photoinduced structural changes have been widely applied in the development of smart materials among other areas, and the design and synthesis of molecular machines was recognized with the 2016 Nobel Prize in Chemistry. Classical photoswitches, including azobenzene and diarylethene, have been extensively studied and facilitate numerous applications. However, the majority of photoswitches necessitate UV light, which limits their use in biological settings and other sensitive environments. Although various strategies have been reported to red-shift absorbance bands to the visible and near-infrared regions, they often lead to diminished switching efficiencies.
Discovered in 2014, the donor-acceptor Stenhouse adduct (DASA) photoswitch absorbs visible to near-infrared light (500–750 nm) to switch from an open form to a closed form (Fig. 1a)2. The absorbance of low-energy light is enabled by significant electron delocalization resulting from a push-pull system connected through a triene bridge. DASAs exhibit negative photochromism, meaning that the open form is highly colored while the closed form is colorless. This characteristic facilitates deeper light penetration in bulk material. Along with appealing optical properties, significant changes in size and charge upon photoswitching make DASAs a prominent photoswitch platform.
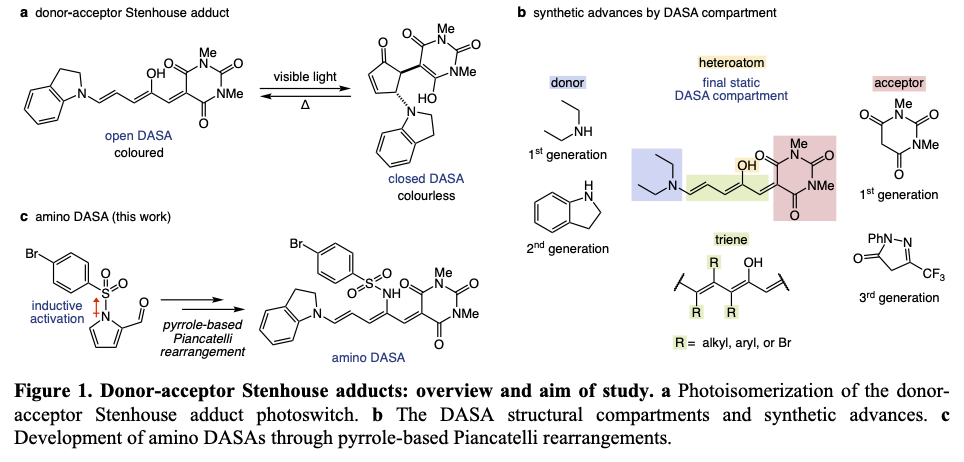
Synthetic modularity of DASA molecules has facilitated structural advances that have expanded their photophysical properties (Fig. 1b)3. Second generation DASAs use aryl amine donors which red-shift absorbance and enable productive photoswitching in polar solvents. Third generation DASAs expand the acceptor scope to more electron withdrawing carbon acids. These acceptors further red-shift absorbance and increase their open-to-closed isomer equilibrium, amplifying switching efficiencies. More recently, triene bridge substitutions have been studied. These substitutions show significant effects on optical properties, and steric tuning has been used to increase closed-to-open thermal reversion rates. Prior to this contribution, the backbone heteroatom remained the final static DASA compartment and had been limited to hydroxy groups. The DASA backbone heteroatom structural compartment originates from the heterocyclic starting material that undergoes an interrupted Piancatelli rearrangement. Difficulty in generating DASA photoswitches with varying heteroatomic properties is connected to related rearrangements dating back to the 1850s4, which were limited to furan-based starting materials.
Development
Motivated by the possibility to further tune the photophysical properties of DASA photoswitches, we sought to derivatize the backbone heteroatom. Therefore, we envisioned that heterocycles bearing non-oxygen heteroatoms can be activated to undergo an interrupted Piancatelli rearrangement. It was previously reported that thiophenes and pyrroles failed to undergo the rearrangement5. Given this precedent and an increased aromatic stability in comparison to furan, we knew that we needed to induce significant electronic activation of pyrroles to produce the desired reactivity. Unlike furan or thiophene, the pyrrole nitrogen possesses an additional bonding orbital that can be leveraged to tune the heterocyclic electronic nature. To this end, we synthesized pyrrole substrates bearing various electron withdrawing groups at nitrogen. After observing hints of reactivity with increasing activation, we found that sulfonyl groups furnish the DASA derivative in 74% yield (Fig. 1c).
Characterization
Having found the optimal reaction conditions and pyrrole substrates to produce amino DASAs, we synthesized DASAs of each generation to study photophysical properties. Initial photoswitching studies with UV-vis spectroscopy show that upon visible light irradiation, amino DASAs have significantly slower absorbance decrease rates in comparison to their hydroxy counterparts. To further interrogate which isomerization step is limiting photoswitching, we used variable-temperature NMR. Here, it is observed that sulfonyl amino DASAs have decreased switching efficiencies due to poor alkene photoisomerization, even after prolonged periods of irradiation with a narrow-band LED. Amino DASA stability studies show that in the absence of reversable photoswitching, the slow absorbance decrease observed with UV-vis spectroscopy is the result of reversion to pyrrole starting materials.
Despite hampered photoswitching, the synthesis of novel sulfonamide backbone heteroatom derivatives enabled the study backbone heteroatom effects on optical properties. In comparison to their hydroxy counterparts, amino DASAs exhibit an approximately 40 nm blue-shifted absorbance with varying shifts depending on the donor or acceptor. Moreover, the sulfonamide group also decreases absorption coefficients and affects the charge distribution of the DASA open form. These results provide insights into the effects of varying electronic contributions from the backbone heteroatom and suggest possible noncovalent interactions between the backbone heteroatom and other structural compartments.
Conclusion
This study has unlocked the final DASA structural compartment and opens the door for future development of other backbone heteroatom derivatives. Efforts to further understand the role of the sulfonamide group on optical properties and photoswitching are ongoing. Additionally, pyrrole-based Piancatelli rearrangements may be expanded beyond DASA synthesis and find utility for the synthesis of other molecules of interest. We look forward to developments in these areas and are excited to share our findings with the community.
References
1. Feringa, B. L. The art of building small: from molecular switches to motors (Nobel Lecture). Chem. Int. Ed. 56, 11060–11078 (2017). DOI: 10.1002/anie.201702979
2. Helmy, S., Leibfarth, F. A., Oh, S., Poelma, J. E., Hawker, C. J. & Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 136, 8169–8172 (2014). DOI: 10.1021/ja503016b
3. Clerc, M., Sandlass, S., Rifaie-Graham, O., Peterson, J. A., Bruns, N., Read de Alaniz, J. & Boesel, L. F. Visible light-responsive materials: The (photo)chemistry and applications of donor-acceptor Stenhouse adducts in polymer science. Chem Soc. Rev. 52, 8245–8294 (2023). DOI: 10.1039/D3CS00508A
4. Verrier, C., Moebs-Sanchez, S., Queneau, Y. & Popowycz, F. The Piancatelli reaction and its variants: recent applications to high added-value chemicals and biomass valorization. Biomol. Chem. 16, 676–687 (2018). DOI: 10.1039/C7OB02962D
5. Noirbent, G., Xu, Y., Bonardi, A.-H., Duval, S., Gigmes, D., Lalevée, J. & Dumur, F. New donor-acceptor Stenhouse adducts as visible and near infrared light polymerization photoinitiators. Molecules 25, 2317 (2020). DOI: 10.3390/molecules25102317
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in