Structural effects of hydrogen treatment at different temperatures on carbon nitride and the metal.
Inspired by Nature: Carbon Nitride Photocatalysts Turning CO2 into Opportunity
Explore the Research
Shibboleth Authentication Request
Due to the increasing importance of reducing pollutant gas emissions, the world's high energy dependence, and the urgent need to develop clean energy production methods without relying on harmful materials, materials science is currently experiencing a remarkable surge. Additionally, pollution is severely impacting nature, contaminating the air we breathe and the water that sustains marine ecosystems. This has created a pressing need to find a material capable of addressing all these challenges.
The goal is clear: to discover a material that is eco-friendly, abundant, non-destructive to natural resources, and capable of generating energy from renewable sources. Alternatively, such a material could also help purify the surrounding air or treat water contaminated by pollutants. Sounds ideal, right? While it may seem utopian, the concept has a surprisingly simple scientific basis and it's precisely what modern materials research is striving to achieve.
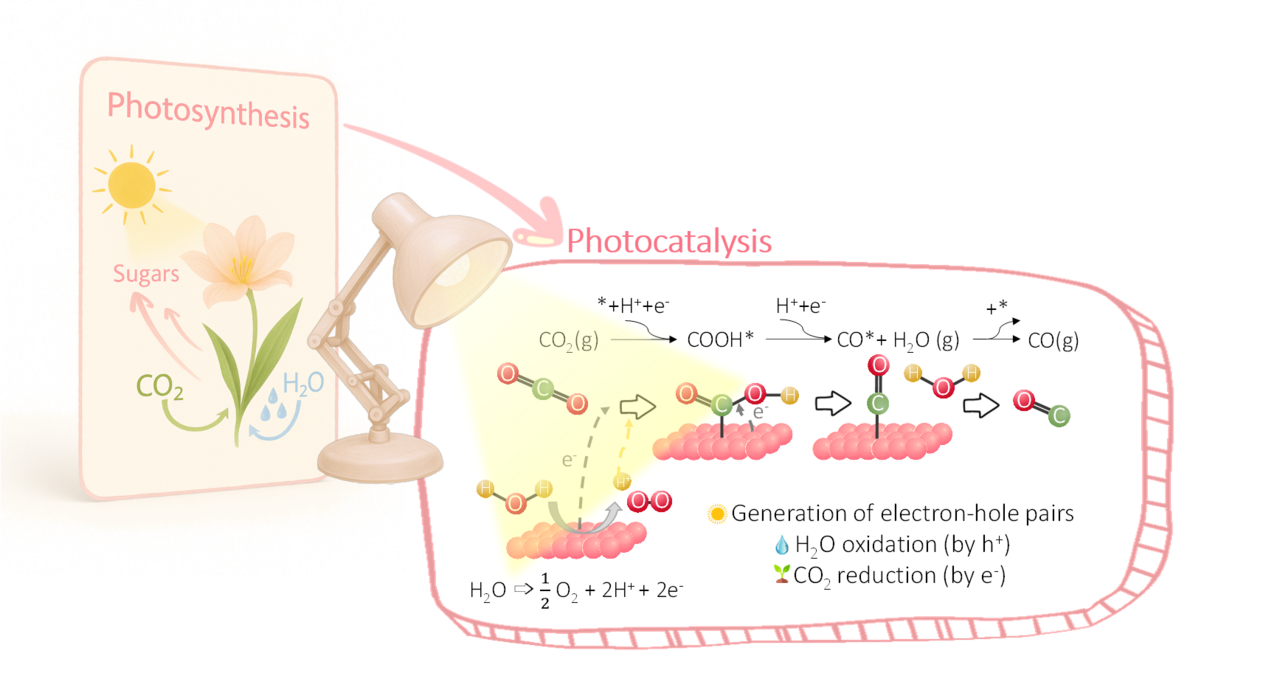
Well, this idea is the foundation of our research. Inspired by nature, we want to imitate the photosynthesis carried out by plants. Plants, through photosynthesis, use sunlight, water, and carbon dioxide to produce their food (glucose) and release oxygen. We seek something similar: to convert CO2 into useful products to generate energy, using a type of material called a semiconductor.
A semiconductor is a material capable of absorbing light, similar to how chloroplasts work in plants. But in our case, we don’t use chlorophyll to transform CO2; instead, we use a material based on carbon and nitrogen called carbon nitride.
So, what makes carbon nitride so special? Can it really convert CO2 into syngas (a mixture of CO and H2) using only sunlight? Can it be synthesized sustainably? Is it affordable? The answer is yes, although with some nuances.
All this can be achieved through a process called photocatalysis. Catalysis is when a material (the catalyst, in our case carbon nitride) helps a chemical reaction occur faster and more easily. Usually, these processes are performed at high temperatures, as this provides the energy needed for the reaction to occur. But in photocatalysis, this energy comes directly from light. The work published in J. CO2 Utiliz. 95 (2025) 103068 highlights a thermally coupled photocatalytic system that makes use of the solar spectrum to hydrogenate CO2 to CO using a double-sided reactor funded by the CATART project.
How do we achieve this? Every chemical reaction simultaneously involves a reduction (gain of electrons) and an oxidation (loss of electrons). Light absorption by carbon nitride generates excited electrons and “holes” (the empty spaces left by those electrons). These electrons and holes participate in the reduction and oxidation of the involved substances.
However, sometimes this electron transfer is complicated because electrons can lose energy or recombine with holes before completing their function, reducing efficiency. Therefore, to increase effectiveness, we have modified carbon nitride by adding small amounts of platinum and altering its internal structure.
In this way, we ensure that CO2 molecules anchor better to the material, generating different types of active sites from nitrogen and carbon vacancies. (A vacancy is an empty spot in the material’s structure where an atom should be, and this absence allows other molecules to bind more easily and facilitates chemical reactions.) Additionally, platinum nanoparticles act as active sites and as a kind of “electron distributor,” preventing electrons from being lost along the way and ensuring they complete their function.
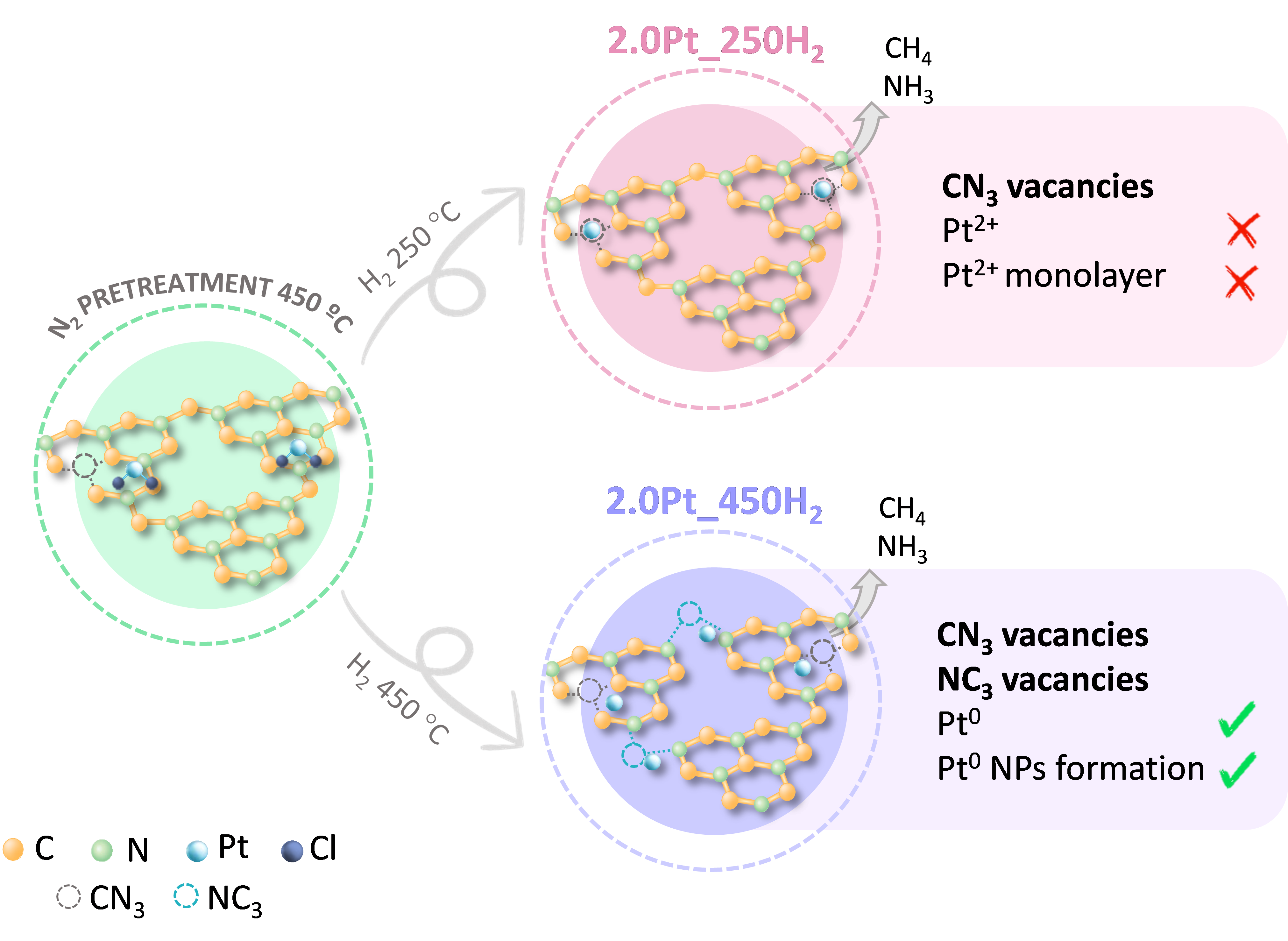
What we discovered in our research is that depending on how we modify the material, we obtain different results. To do this, we impregnated the surface of carbon nitride with platinum nanoparticles suspended in water, then thermally treated it in a furnace under nitrogen and hydrogen atmospheres at different temperatures.
We found that when treated with hydrogen at 250 °C or 450 °C, the photocatalyst exhibits different behaviors. On one hand, vacancies are generated in the carbon nitride, and on the other, the platinum nanoparticles adopt different forms. At lower temperatures, platinum forms a Pt2+ monolayer on the photocatalyst surface; at 450 °C, platinum nanoparticles disperse as metallic particles.
We performed tests in a gas-phase photocatalytic reactor (although it is common in photocatalysis to work in liquid phase, we chose gas phase to facilitate industrial scalability). We used flows of CO2 and hydrogen (yes, hydrogen and not water, but we have to start somewhere) and combined irradiation with a solar simulator in a pressurized photoreactor.
We discovered that materials treated at higher temperatures showed higher carbon monoxide productivity (a product with high energy value) and were thus more efficient. Additionally, we observed that the reaction improved with the combination of light and temperature. This allowed us to conclude that material treatment is crucial and that coupling light and thermal energy is a viable strategy to optimize the performance of these reactions.
Now, our goal is to advance towards reducing CO2 using water directly and, at the same time, producing hydrogen from water. We start with a simpler approach to understand more complex processes, because science always progresses step by step.
Although these innovative ideas may seem difficult to achieve, it is essential to remember that every great advance begins from an imperfect starting point. Perhaps the process is not yet the most efficient and much remains to be improved, but this is the first step that paves the way for future improvements and a more sustainable future.
Follow the Topic
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in